Published online Apr 28, 2009. doi: 10.3748/wjg.15.2027
Revised: March 17, 2009
Accepted: March 24, 2009
Published online: April 28, 2009
AIM: To investigate the incidence of incidental gastrointestinal stromal tumor (GIST) and its etiopathogenesis.
METHODS: From January 1, 2000 to December 31, 2007, 13 804 cases of gastrointestinal epithelial malignant tumor (EMT) and 521 cases of pancreatic adenocarcinoma (PAC) were successfully treated with surgery at the Department of General Surgery and the Department of Thoracic Surgery, West China Hospital, Sichuan University, China. The clinical and pathologic data of 311 cases of primary GIST, including 257 cases with clinical GIST and 54 cases of incidental GIST were analyzed.
RESULTS: Of the 311 patients, 54 had incidental GIST, accounting for 17.4%. Of these tumors, 27 were found in 1.13% patients with esophageal squamous cell carcinoma (ESCC), 22 in 0.53% patients with gastric adenocarcinoma (GAC), 2 in 0.38% patients with PAC, 2 in 0.03% patients with colorectal adenocarcinoma, and 1 in one patient with GAC accompanying ESCC, respectively. Patients with incidental GIST presented symptoms indistinguishable from those with EMT. All incidental GIST lesions were small in size, and the majority had a low mitotic activity while only 1.9% (5/257) of clinical GIST lesions had a high risk.
CONCLUSION: Incidental GIST may occur synchronously with other tumors and has a high prevalence in males. Surgery is its best treatment modality.
- Citation: Liu YJ, Yang Z, Hao LS, Xia L, Jia QB, Wu XT. Synchronous incidental gastrointestinal stromal and epithelial malignant tumors. World J Gastroenterol 2009; 15(16): 2027-2031
- URL: https://www.wjgnet.com/1007-9327/full/v15/i16/2027.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2027
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of gastrointestinal (GI) tract, probably arising from precursor interstitial cells of Cajal. Significant advances have been made in symptomatic GIST in the last two decades[12]. However, little is known about the incidental GIST detected during examinations or surgery for other reasons. Its clinicopathologic characteristics are unclear. Many cases of synchronous or asynchronous GIST with other tumors have been reported as single cases[3–6]. We discovered 54 cases of incidental GIST during surgery for epithelial malignant tumor (EMT). This study was to investigate the incidence of incidental GIST and its etiopathogenesis.
From January 1, 2000 to December 31, 2007, 13 804 cases of gastrointestinal EMT and 521 cases of pancreatic adenocarcinoma (PAC) were successfully treated with surgery at the Department of General Surgery and the Department of Thoracic Surgery, West China Hospital, Sichuan University, China. Gastrointestinal EMT cases included 2382 cases of esophageal squamous cell carcinoma (ESCC), 35 cases of esophageal adenocarcinoma (EAC), 4168 cases of gastric adenocarcinoma (GAC), 329 cases of small intestinal adenocarcinoma (SAC), and 6890 cases of colorectal adenocarcinoma (CRA). During this period, 311 cases of primary GIST (121 females, 190 males) were identified in our center, including 257 cases of clinical GIST and 54 cases of incidental GIST.
Hospital records of patients with incidental GIST were reviewed. Each patient was followed up by telephone or mail. Histopathologic features of primary GIST were evaluated by two experienced pathologists, blinded to their respective findings and patient outcomes, at the Department of Pathology, West China Hospital. The largest diameter of tumor was recorded. In patients with multiple GIST lesions, only the largest GIST lesion was included in pathological analysis. The risk category for GIST was defined by assessing the tumor size and mitotic count following the consensus guidelines of the National Institutes of Health-(NIH-NCI) workshop[7]. In addition to the assessment of CD117 in tumor cells, reactions with CD34, SMA, and S-100 proteins were also studied. Immunohistochemical examination of these proteins was performed on tumor tissues embedded in paraffin with DAKO (Glostrup, Denmark) antibodies according to the manufacturer’s instructions.
Categorical variables were compared by χ2 test or by Fisher’s exact test where applicable. Survival analysis was performed using the Kaplan-Meier method. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Of the 311 patients, 54 had incidental GIST, accounting for 17.4%. Among these tumors, 27 were found in 1.13% patients with ESCC, 22 in 0.53% patients with GAC, 2 in 0.38% patients with PAC, 2 in 0.03% patients with CAC, and 1 in one patient with GAC accompanying ESCC, respectively.
The median age of the 54 cases of incidental GIST was 63 years (range, 44-79 years). Interestingly, 48 of them (88.9%) were males, and 6 (11.1%) were females (P < 0.001). The patients presented symptoms of EMT without specific clinical manifestations indicative of GIST. Among the 54 patients, only a submucous lesion in gastric fundus, 2.5 cm in diameter, was preoperatively detected in 1 patient with GAC by gastroscopy, and a single-lesion was postoperatively detected in 4 patients by specimen examination. A total of 58 incidental GIST lesions were discovered in the 54 patients, including 51 single-lesions, 2 double-lesions, and 1 triple-lesion. A total of 90.7% incidental GIST lesions occurred in stomach, 3.6% in esophagus, 1.9% in terminal ileum, 1.9% in colon and 1.9% in omentum, respectively. The most common sites were the gastric fundus and body. In our series, 4 cases with a unique coexistence style (esophageal GIST + ESCC: 2, gastric GIST + ESCC + GAC: 1, colonic GIST + CRA: 1) have not been reported previously. The location of 54 incidental GIST lesions and their corresponding EMT lesions are shown in Table 1.
| EMT | Patients (n) | Median age | Gender (M/F) | Incidental GIST site (No. of patients) | |||||||
| Gastric cardia | Gastric fundus | Gastric body | Gastric antrum | Esophagus | Terminal ileum | Colon | Omentum | ||||
| GAC | 22 | 64.5 (45-79) | 19/3 | 1 | 7 | 13 | 1 | - | - | - | - |
| ESCC | 27 | 63 (44-77) | 24/3 | 1 | 3 | 19 | 1 | 2 | - | - | 1 |
| GAC + ESCC | 1 | 79 | 1/0 | - | - | 1 | - | - | - | - | - |
| CRA | 2 | 57.5 (54-61) | 2/0 | - | - | - | - | - | 1 | 1 | - |
| PAC | 2 | 67.5 (65-70) | 2/0 | - | 1 | 1 | - | - | - | - | - |
| Total | 54 | 63 (44-79) | 48/6 | 2 | 11 | 34 | 2 | 2 | 1 | 1 | 1 |
Of the incidental GIST lesions, 37 (68.5%) were of spindle-cell morphology, 9 (16.7%) epithelioid morphology, and 8 (14.8%) a mixed histological type. Immunohistochemical staining showed that 50 cases (92.6%) and 52 cases (96.3%) of incidental GIST were positive for CD117 and CD34, respectively. None of them was proven to have a metastasis of GIST, while 29 cases were confirmed with metastasis derived from EMT. Incidental GIST was small in size. The majority (90.7%) had a low mitotic activity and a very low risk, while only 1.9% cases of clinical GIST had a very low risk (P < 0.001), and 38.5% had a high risk with a marked mitotic activity (Table 2).
| GIST | Patients (n) | Gender (M/F) | Median age in yr (range) | Tumor site (No. of patients) | Tumor size (cm) | Risk patients, n (%) | ||
| Median | Mean | Range | ||||||
| Incidental GISTs | 54 | 48/6 | 63 (44-79) | Gastric (49), esophagus(2), ileum(1), colon (1), omentum (1) | 0.8 | 0.9 | 0.2-2.5 | VL: 49 (90.7); L: 5 (9.3) |
| Clinical GISTs | 257 | 142/115 | 57 (22-87) | Gastric (147), duodenum (10), jejunum-ileum (57), colon (25), rectum (3), anal canal (3), mesenterium (6), omentum (4), pancreatic (2) | 7.5 | 6.2 | 1.5-30.0 | VL: 5 (1.9); L: 86 (33.5); Int: 67 (26.1); H: 99 (38.5) |
| Total | 311 | 190/121 | 61 (22-87) | Gastric (196), esophagus(2), duodenum (10), jejunum-ileum (58), colon (26), rectum (3) anal canal (3), mesenterium (6), omentum (5), pancreas (2) | 6.3 | 5.5 | 0.2-30.0 | VL: 54 (17.4); L: 91 (29.3); Int: 67 (21.5); H: 99 (31.8) |
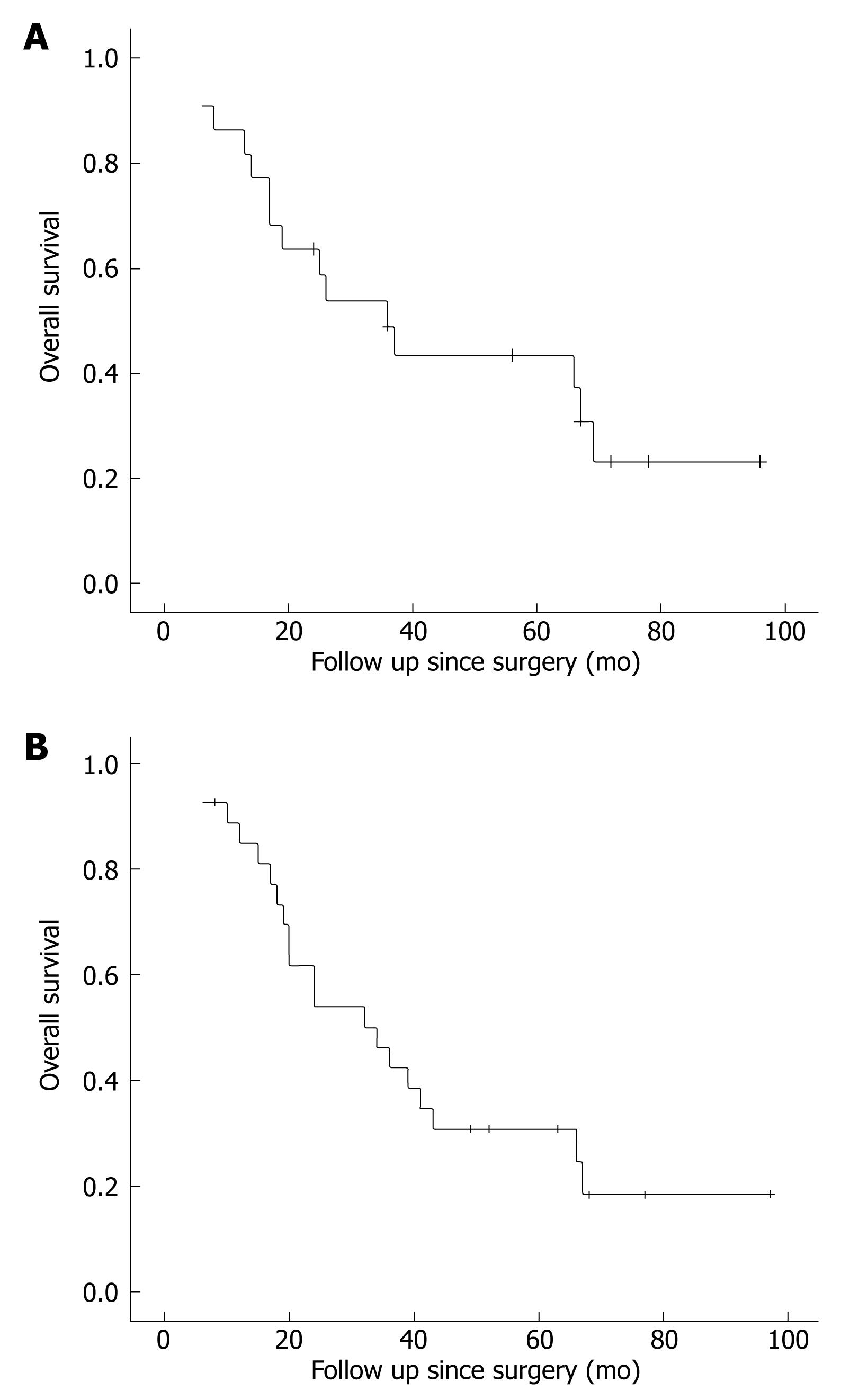
All the GAC patients received radical excision (distal gastrectomy for 3, proximal gastrectomy for 2, total gastrectomy for 12, esophagogastrectomy for 5). All the ESCC patients including the patient with triple tumors underwent esophagogastrectomy. The two PAC patients underwent duodenopancreatectomy and distal pancreatectomy, respectively, with local gastrectomy. Right and left hemicolectomy was performed for the two CRA patients, respectively. Thirty-four out of the 54 patients received either adjuvant chemotherapy and/or radiotherapy after operation. None of them received oral Imatinib mesylate (Glivec) treatment. On September 1, 2008, four of the patients were alive while 50 died of recurrence or distal metastasis of other malignancies. The remaining two patients died of other causes. Recurrent GIST was not found during the survival period of all dead patients, and the follow-up time of the remaining four. The overall 5-year survival rate of the 22 patients with GAC and incidental GIST was 31.8%, with a median survival time of 36 mo (Figure 1A). The 5-year survival rate of the 27 patients with ESCC and incidental GIST was 22.2%, with a median survival time of 32 mo (Figure 1B). The average survival time of the two PAC and two CRA patients was 26 mo and 52 mo, respectively, and the survival time of the patients with triple tumors was 47 mo.
In our series, incidental GIST occurred simultaneously with EMT in 17.4% (54/311) of the GIST patients, which is higher than the reported incidence (14%)[8]. However, assessment of the actual incidence of incidental GIST with EMT is difficult, because the data are only based on patients who have been surgically treated, whereas EMT patients managed with non-surgical measures are unaccounted for. Moreover, during examination or surgery, identification of GIST is incidental rather than intentional, and many lesions are missed as a result.
Notably, in addition to those with EMT, many synchronous and asynchronous cases of GIST with non-epithelial tumors have been reported, such as osteosarcoma, Burkitt’s lymphoma, plasmocytoma, neuroblastoma, somatostatinoma, chronic lymphatic leukemia, lipoma and ectopic pancreas[49–13]. Synchronous incidental GIST and non-tumorous diseases have been reported, such as ulcerative colitis, Meckel’s diverticulum, rapidly progressive glomerulonephritis, HIV carriers, and Crohn’s disease[514–17]. Sanchez et al[18] reported that incidental gastric GIST is found in 0.8% of patients undergoing laparoscopic Roux-en-Y gastric bypass surgery for obesity. Kawanowa et al[19] showed that microscopic GIST can be found in 35% of stomach- resected patients with gastric cancer. It has been shown that microscopic GIST can be found in 10% of patients undergoing surgery for esophageal carcinoma[20]. Especially, incidental GIST has also been detected in 0.2% of all autopsies, accounting for 10% of all patients with primary GIST[21]. These findings suggest that incidental GIST may occur synchronously with other diseases more frequently than expected, and the incidence of incidental GIST might be much higher than that of clinical GIST.
Particular attention has been paid to clinical GIST because of its striking symptoms such as gastrointestinal bleeding, pain, dyspepsia, abdominal mass and obstruction[2223]. On the contrary, incidental GIST may emerge asymptomatically, and even if symptomatically, the symptoms may often be vague and nonspecific[18]. In our study, all the 54 patients presented symptoms indistinguishable from those of EMT, which might have been overlooked because of the progressing symptoms of EMT such as severe dysphagia, weight loss, abdominal pain and anemia. The size of incidental GIST was small, and the majority (90.7%) of them had a very low risk. Also, only a few reports are available on incidental GIST with a high risk[212425]. In this study, only 1.9% of clinical GIST lesions had a very low risk, and 38.5% had a high risk, indicating that GIST is malignant. Perhaps, incidental GIST might have emerged later than EMT, or their development may have been depressed by EMT through mechanisms which are yet to be studied.
Generally, the preoperative detection rate of incidental tumors is very low. In this study, except for two patients with PAC, the other patients received endoscopic examinations preoperatively, yet only one GIST lesion was found. Difficulty in detecting the lesion might be attributed to its small size and intramural location. Incidental GIST, if detected at CT or MRI, is often mistaken for metastatic lymph nodes derived from EMT. Therefore, radiological examination is minimally helpful for its diagnosis. As a result, the endoscopist and surgeon should take the major responsibility of detecting incidental GIST. Incidental GIST occurs most commonly in stomach, esophagus, small bowel, colon and omentum. Consistent with the reported findings[19], incidental GIST was observed in gastric fundus and body in the present study. Careful assessment of the hotspot (i.e. the upper portion of stomach) is important for both endoscopist and surgeon.
Interestingly, we found that there was a significant difference of the incidence of incidental GIST in male and female patients. Because of the unclear pathogenesis of incidental GIST, we cannot explain this finding. Further studies are needed on the gene expression in primary tumor cells from male and female patients and signal transduction may also provide us with some clues to this question.
In the absence of prospective control studies, whether resection of incidental GIST lesions helps to improve the quality of life and/or the survival rate of EMT patients remains unclear. There are two major concerns for incidental GIST if missed during operation for other tumors. First, residual GIST lesions may progress to invasive disease and cause intestinal obstruction and/or life-threatening gastrointestinal hemorrhage because the malignant potential is unpredictable based on gross appearance alone. Second, a residual incidental GIST may be mistaken for the relapse or metastasis of a previously removed neoplasm, which may result in inappropriate treatment of patients in follow-up after operation. Therefore, an en bloc resection with other tumors or an additional local resection with adequate margins has been recommended by surgeons[61825]. Making surgeons aware of this will help to correct surgical procedures, and ultimately improve the quality of life and avoid inappropriate treatment of patients during follow-up after operation.
Common carcinogenic agents, which result in a simultaneous proliferation of different cell lines (epithelial and stromal cells), may be involved in the development of incidental GIST as a mere coincidence. In this study, males with primary GIST were more likely to have a synchronous tumor than females (P < 0.001). Synchronous tumors may have a high prevalence in males. Simultaneous neoplastic proliferation of epithelial and stromal cells might be stimulated by the same carcinogenic factors, such as Helicobacter pylori infections, germline mutations, and exposure to ionizing radiation[6242627]. To clarify possible common carcinogenic agents against synchronous tumors, further studies are needed.
In conclusion, incidental GIST coexists with EMT at a higher incidence than expected. Surgeons are advised to be alert against possible primary GIST accompanying other tumors.
Gastrointestinal stromal tumor (GIST) is one of the most common tumors in gastrointestinal (GI) tract, probably arising from precursor cells that serve as a pacemaker to trigger gut contraction. It may exist alone with clinical manifestations or coexist with other diseases. The former is usually diagnosed by its clinical presentations and called clinical GIST, while the latter is usually found during examination or surgery for other diseases and called incidental GIST.
Clinical GIST has been extensively studied in the past twenty years. Many cases of GIST existing alone or coexisting with other diseases have been reported, but GIST coexisting with other GI tumors has only been reported as single cases. It is necessary to conduct a comprehensive study with a large sample size to determine its incidence and features.
For the first time, the authors report an extensive study on incidental GIST coexisting with other GI tumors. This study revealed some important and interesting information regarding incidental GIST coexisting with other GI tumors. Firstly, they found that incidental GIST coexisted most frequently with esophageal and gastric tumor (1.13% and 0.53% respectively), and least with colorectal tumor (0.03%). Secondly, the majority of clinical GISTs had a moderate or a high risk. In contrast, the majority of incidental GISTs had a very low risk. Thirdly, the incidence of incidental GIST was significantly higher in male than in female patients (88.9% vs 11.1%). Finally, this study also provided the statistics for age, survival time and prognosis of studied patients and outlined the other features of incidental GIST, such as the number of lesions, lesion location and cellular morphology, etc.
The incidence of incidental GIST coexisting with other GI tumors is much higher than expected. However, without specific manifestations, preoperative detection of incidental GIST is difficult. Residual GIST lesions may progress to invasive diseases, cause intestinal obstruction and/or life-threatening gastrointestinal hemorrhage. In addition, residual incidental GIST may be mistaken for the relapse or metastasis of previously removed tumors, resulting in inappropriate treatment of patients during follow-up after operation. A careful inspection for GIST is highly recommended during surgery for GI tumors.
GIST is one of the tumors in the GI tract, probably arising from precursor cells that serve as a pacemaker to trigger gut contraction. GI epithelial malignant tumor (EMT) refers to a tumor arising from the surface cells of the GI tract.
This article is the first report to present the incidence of incidental GIST accompanying gastrointestinal EMT. In this study, the authors evaluated the incidental GIST and its clinical significances. The title of the paper reflects the major contents of the article. The abstract gives a clear delineation of the research background. Results and discussion are well organized. The conclusion is reliable and valuable.
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. |
| 3. | Liu SW, Chen GH, Hsieh PP. Collision tumor of the stomach: a case report of mixed gastrointestinal stromal tumor and adenocarcinoma. J Clin Gastroenterol. 2002;35:332-334. |
| 4. | Au WY, Wong WM, Khoo US, Liang R. Challenging and unusual cases: Case 2. Concurrent gastrointestinal stromal tumor and Burkitt's lymphoma. J Clin Oncol. 2003;21:1417-1418. |
| 5. | Pfeffel F, Stiglbauer W, Depisch D, Oberhuber G, Raderer M, Scheithauer W. Coincidence of Crohn's disease and a high-risk gastrointestinal stromal tumor of the terminal ileum. Digestion. 1999;60:363-366. |
| 6. | Lin YL, Tzeng JE, Wei CK, Lin CW. Small gastrointestinal stromal tumor concomitant with early gastric cancer: a case report. World J Gastroenterol. 2006;12:815-817. |
| 7. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. |
| 8. | Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, Cebulski W, Slodkowski M, Wasiutynski A, Krasnodebski IW. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360-5362. |
| 9. | Ruka W, Rutkowski P, Nowecki Z, Nasierowska-Guttmejer A, Debiec-Rychter M. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST). Med Sci Monit. 2004;10:LE13-LE14. |
| 10. | Johnston DL, Olson JM, Benjamin DR. Gastrointestinal stromal tumor in a patient with previous neuroblastoma. J Pediatr Hematol Oncol. 2001;23:255-256. |
| 11. | Agaimy A, Wuensch PH. Gastrointestinal stromal tumours in patients with other-type cancer: a mere coincidence or an etiological association? A study of 97 GIST cases. Z Gastroenterol. 2005;43:1025-1030. |
| 12. | Usui M, Matsuda S, Suzuki H, Hirata K, Ogura Y, Shiraishi T. Somatostatinoma of the papilla of Vater with multiple gastrointestinal stromal tumors in a patient with von Recklinghausen's disease. J Gastroenterol. 2002;37:947-953. |
| 13. | Teke Z, Kabay B, Kelten C, Yilmaz M, Duzcan E. Ectopic pancreas of the gastric antrum contiguous to a gastrointestinal stromal tumor manifesting as upper gastrointestinal bleeding: report of a case. Surg Today. 2007;37:74-77. |
| 14. | Grieco A, Cavallaro A, Potenza AE, Mulè A, Tarquini E, Miele L, Gasbarrini G. Gastrointestinal stromal tumor (GIST) and ulcerative colitis. J Exp Clin Cancer Res. 2002;21:617-620. |
| 15. | de la Morena López F, Fernández-Salazar L, Velayos B, Aller R, Juárez M, González JM. [Meckel's diverticulum and gastrointestinal stromal tumor: an unusual association]. Gastroenterol Hepatol. 2007;30:534-537. |
| 16. | Nakaya I, Iwata Y, Abe T, Yokoyama H, Oda Y, Nomura G. Malignant gastrointestinal stromal tumor originating in the lesser omentum, complicated by rapidly progressive glomerulonephritis and gastric carcinoma. Intern Med. 2004;43:102-105. |
| 17. | Padula A, Chin NW, Azeez S, Resetkova E, Andriko JA, Miettinen M. Primary gastrointestinal stromal tumor of the esophagus in an HIV-positive patient. Ann Diagn Pathol. 2005;9:49-53. |
| 18. | Sanchez BR, Morton JM, Curet MJ, Alami RS, Safadi BY. Incidental finding of gastrointestinal stromal tumors (GISTs) during laparoscopic gastric bypass. Obes Surg. 2005;15:1384-1388. |
| 19. | Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527-1535. |
| 20. | Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. "Seedling" mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629-1635. |
| 21. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. |
| 22. | de Francisco R, Díaz G, Cadahia V, Velázquez RF, Giganto F, González O, Rodrigo L. Lower GI bleeding secondary to a stromal rectal tumor (rectal GIST). Rev Esp Enferm Dig. 2006;98:387-389. |
| 23. | Nowain A, Bhakta H, Pais S, Kanel G, Verma S. Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol. 2005;20:818-824. |
| 24. | Aksoy NH, Cevikol C, Ogüs M, Elpek GO, Gelen T. Adenocarcinoma arising in villous adenoma of the ampulla of Vater with synchronous malignant gastrointestinal stromal tumour of the duodenum: a case report. J Clin Pathol. 2004;57:1118-1119. |
| 25. | Maiorana A, Fante R, Maria Cesinaro A, Adriana Fano R. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682-686. |
| 26. | Kaffes A, Hughes L, Hollinshead J, Katelaris P. Synchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomach. J Gastroenterol Hepatol. 2002;17:1033-1036. |
| 27. | Miller PR, Jackson SL, Pineau BC, Levine EA. Radiation-induced gastrointestinal stromal sarcoma of the esophagus. Ann Thorac Surg. 2000;70:660-662. |