Published online Apr 28, 2009. doi: 10.3748/wjg.15.2009
Revised: December 29, 2008
Accepted: January 5, 2009
Published online: April 28, 2009
AIM: To investigate the effect of Lianshu preparation on lipopolysaccharide (LPS)-induced diarrhea in rats.
METHODS: A diarrhea model was established in Sprague Dawley rats via injection of 1 mL of 30 mg/kg LPS. A total of 40 rats were randomly divided into normal group, LPS group, LPS + Lianshu group, LPS + berberine group (n = 10 in each group). Their intestinal mucosal barrier and frequency of diarrhea were observed. Levels of glucose, serum Na+, K+, Cl- and hematocrit, plasma nitrogen monoxide (NO), diamine oxidase (DAO), and D (-)-lactate were measured. The number of IgA+ plasma cells in small intestine was detected and SIgA levels in the intestinal fluid were measured. The antipyretic activity of Lianshu preparation in rats was evaluated using Brewer’s yeast-induced pyrexia (10 mL/kg of 20% aqueous suspension). Acetaminophen (250 mg/kg, intragastric administration, bid) was used as a standard drug for comparison. Temperature was recorded 1 h before and 6 h after Brewer’s yeast injection. Finally, small intestinal transmission in mice treated with Lianshu was detected after intraperitoneal injection of methyl prostigmin (2 mg/kg). Atropine (10 g/kg) was used as a control. The ink content in intestine was determined and the total length of intestine was measured.
RESULTS: The frequency of diarrhea was higher in LPS group than in LPS + Lianshu group and LPS + berberine group (36.70 ± 5.23 vs 28.50 ± 4.06 and 32.70 ± 9.30 respectively, P < 0.01), and lower in LPS + Lianshu group than in LPS + berberine group (P = 0.03). The levels of Na+, glucose, Cl-, K+ were significantly lower in LPS + Lianshu group than in LPS + berberine group (140.35 ± 3.19 mmol/L vs 131.99 ± 4.86 mmol/L, 8.49 ± 1.84 mmol/L vs 6.54 ± 2.30 mmol/L, 106.29 ± 4.41 mmol/L vs 102.5 ± 1.39 mmol/L, 5.08 ± 0.66 mmol/L vs 4.32 ± 0.62 mmol/L respectively, P < 0.05). The level of hematocrit was lower in LPS + Lianshu group than in LPS + berberine group (0.50% ± 0.07% vs 0.59% ± 0.10% respectively, P < 0.05). The plasma levels of NO, DAO and D (-)-lactate were higher in LPS group than in normal group (79.74 ± 7.39 &mgr;mol/L vs 24.94 ± 3.38 &mgr;mol/L, 2.48 ± 0.42 &mgr;/mL vs 0.82 ± 0.33 &mgr;/mL, 5.63 ± 0.85 &mgr;g/mL vs 2.01 ± 0.32 &mgr;g/mL respectively, P < 0.01), and lower in LPS + Lianshu group than in LPS + berberine group (48.59 ± 4.70 &mgr;mol/L vs 51.56 ± 8.38 &mgr;mol/L, 1.43 ± 0.53 &mgr;mol/mL vs 1.81 ± 0.42 &mgr;mol/mL, 4.00 ± 0.54 &mgr;g/mL vs 4.88 ± 0.77 &mgr;g/mL respectively, P < 0.05). The morphology of the intestinal mucosa showed destroyed villi in LPS group and atrophied intestinal mucosa in other groups. The pathological intestinal mucosal changes were less in LPS + Lianshu group than in LPS group. The number of IgA+ plasma cells and amount of SIgA were higher in LPS + Lianshu group than in LPS group (1.16 ± 0.19/&mgr;m2vs 1.09 ± 0.28/&mgr;m2, P = 0.026; 0.59 ± 0.12 mg/L vs 0.15 ± 0.19 mg/L respectively, P = 0.000). Lianshu had counteractive effects on yeast-induced pyrexia and enterokinesia in rats.
CONCLUSION: Lianshu preparation has therapeutic effects on LPS-induced diarrhea and enterokinesia in rats.
- Citation: Liu J, Wan R, Xu XF, Wang XP, Yang WJ, Xia YJ, Liu H, Yan QL, Yan DX, Guo CY. Effect of Lianshu preparation on lipopolysaccharide-induced diarrhea in rats. World J Gastroenterol 2009; 15(16): 2009-2015
- URL: https://www.wjgnet.com/1007-9327/full/v15/i16/2009.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2009
Infectious diarrhea is often caused by Gram-negative bacteria such as Escherichia coli. These organisms contain lipopolysaccharide (LPS). Antibiotics developed in the 1940s have saved millions of people’s life. However, because of the widespread and inappropriate use of antibiotics, some bacterial strains become antibiotic-resistant. Antibiotic-resistant bacteria and side effects of antibiotics severely threaten the general welfare and health of people. Folk herbal drugs have been extensively studied in recent years for the treatment of acute diarrhea. This study was to investigate the effect of Lianshu preparation on LPS-induced diarrhea in rats.
Eighty adult female and male Sprague-Dawley rats, weighing 230-280 g (Experimental Animal Breeding Center, Fudan University), and thirty 5-wk-old female and male Kunming mice, weighing 18-20 g, were used in this study following the guidelines of the Animal Care Committee of Institute of Chinese Medicine, Tongji University. The animals were housed at 22 ± 2°C with a humidity of 55% ± 5% in a 12-h light/dark cycle for at least 1 wk prior to use, with free access to laboratory chow and tap water.
Coptis chinensis, atractylodis, pulsatillae and portulacae, provided by Leiyunshang Company, were treated twice at 100°C for 2 h, divided into 1:5:5:5 proportions, then treated at 100°C for 1 h, filtered and concentrated to a relative density of 1.25 (60°C), and finally, desiccated. The effective constituents of Lianshu preparation were determined by Ley’s Company (Shanghai, China). Lipopolysaccharide from Escherichia coli serotype 0128:B12 was purchased from Sigma Aldrich (St Louis, MO, USA). Nitrogen monoxide (NO) assay kit was provided by Jiancheng Bionic Research Center (Nanjing, China). Diamine oxidase (DAO) standard preparation, O-dianisidine, horseradish peroxidase, 1,5-pentanediamine-di-hydrochloride, D-lactate standard preparation, NAD- aminoacetic acid, and D-lactic dehydrogenase were purchased from Sigma Aldrich (St Louis, MO, USA). Anti-rat IgA mAb was purchased from Santa Crus Company (USA) and SIgA assay kit was purchased from Bethyl Labs Inc. (USA).
Rats were randomly divided into normal group, LPS group, LPS + Lianshu group, LPS + berberine group (n = 10 in each group). Before exposure to LPS, rats in normal and LPS groups were treated with distilled water, rats in LPS + Lianshu group were given Lianshu preparation (1.8 g/kg), rats in LPS + berberine group received berberine (0.2 g/kg) twice a day for 3 d. The rats were allowed to have water alone for 12 h. One hour after the last administration of drugs, rats in all groups apart from those in the normal group were treated with LPS (30 mg/kg).
After treated with LPS, rats in each cage had a filter paper couch. Filter paper was changed once an hour for 4 h. The frequency of diarrhea was determined by counting the number of feces deposits on the filter paper.
Four hours later, 8 mL of blood was drawn from the abdominal aorta of each rat after anesthetized with 10 mg/kg intraperitoneal pentobarbital. Of the 8 mL blood, 2 mL was treated with heparin for erythrocrit analysis, 2 mL was centrifuged at 3000 ×g for 15 min at 4°C. Blood glucose and serum Na+, K+, Cl- levels were measured.
Four milliliters of blood was centrifuged at 3000 ×g for 15 min at 4°C. Plasma was collected to measure NO at 550 nm as previously described[1]. DAO and D (-)-lactate levels were measured by spectrophotometry at 436 nm[2] and enzyme-linked ultraviolet spectrophotometry[3], respectively. The absorbance at 340 nm was recorded.
After humane killing, a 5-cm long section of ileum 10 cm below the Treitz ligament was removed, dissected longitudinally, fixed in 100 mL/L formalin, embedded in paraffin, stained with hematoxylin and eosin, and observed under a light microscope.
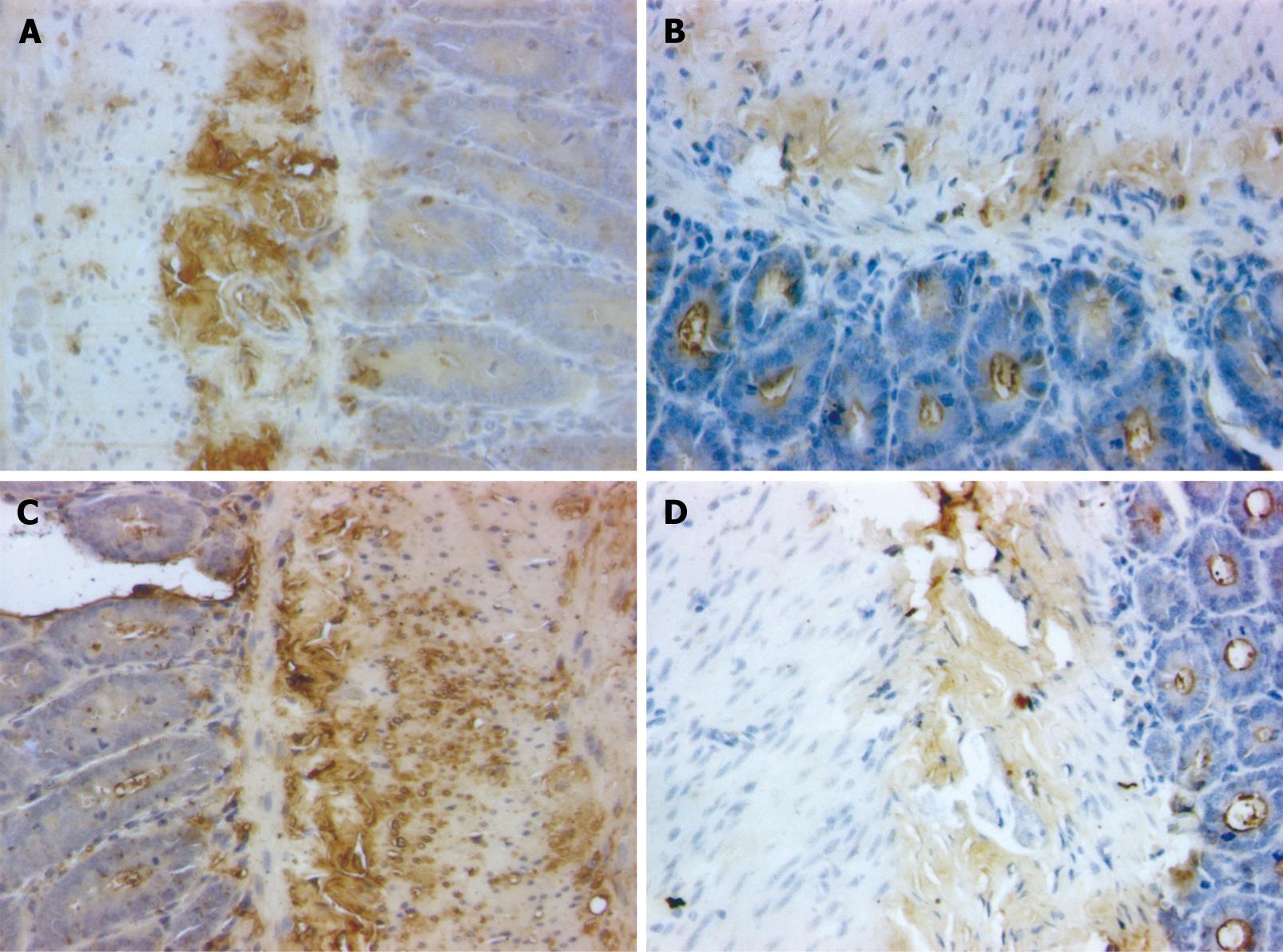
ABC immunohistochemistry staining of tissue paraffin sections was performed. The brown stained IgA + plasma cells were detected and analyzed using medical image analysis software (Taimeng Technology Co. Chengdu, China).
A small intestine segment about 5 cm long, 15 cm below the Treitz ligament, was removed. Intestinal fluid was collected by passing 5 mL PBS and 0.02% sodium into the small intestine. The first intestinal washing removed 85%-90% of total IgA, and the variability of samples was less than 5%. The washed out material was centrifuged at 3000 ×g for 10 min at 4°C. The supernatant was harvested and stored at -70°C. Total IgA was determined by ELISA.
Antipyretic activity of Lianshu preparation in rats was evaluated using Brewer’s yeast-induced pyrexia. Forty rats were divided into 4 groups. Rats in control group were given sodium chloride (2 mL) and rats in experimental groups were given Lianshu preparation (1.8 g/kg), twice a day before yeast was injected. Acetaminophen (250 mg/kg, bid) was used as a standard drug to compare the antipyretic action of Lianshu preparation. One hour after the last administration of drugs, fever was induced by hypodermic injection of a 20% suspension of 10 mL/kg Brewer’s yeast and rectal temperature was recorded 1 h before and 6 h after injection of Brewer’s yeast. The antipyretic activity of Lianshu preparation in rats was assayed.
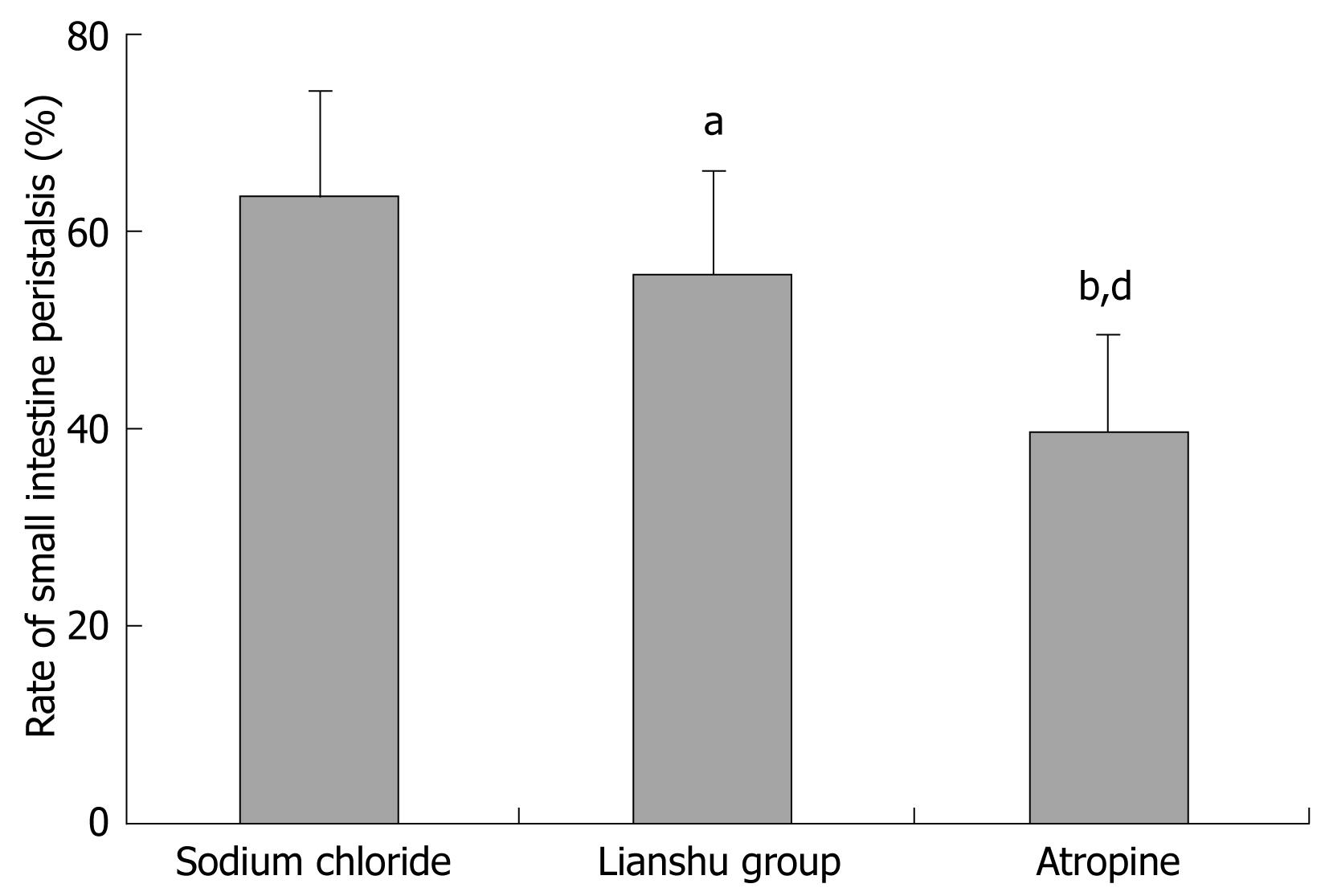
Thirty Kunming mice, after 12 h fasting, were divided into sodium chloride group, Lianshu preparation group and atropine group (n = 10). Mice in the sodium chloride group received 0.5 mL sodium chloride by intragastric administration, twice a day for 1 d. Mice in the Lianshu preparation group received 1.8 g/kg Lianshu preparation, twice a day for 1 d. Mice in the atropine treatment group received 10 g/kg intraperitoneal atropine, twice a day for 1 d. All mice were given 2 mg/kg methyl prostigmin followed by 0.1 mL ink after 10 min, and killed by cervical vertebral dislocation with their belly cut open to collect intestines. The effect of Lianshu preparation on small intestinal transmission in mice was detected.
Data were expressed as mean ± SE and analyzed by analysis of variance and Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
No diarrhea and loose stools occurred in rats of the normal group. The fur of rats was smooth and the rats were active. Rats in the other groups that received LPS drank more water and had loose stools. The fur of rats was fluffy, and the rats were affected by lassitude. The respiration of rats was rapid.
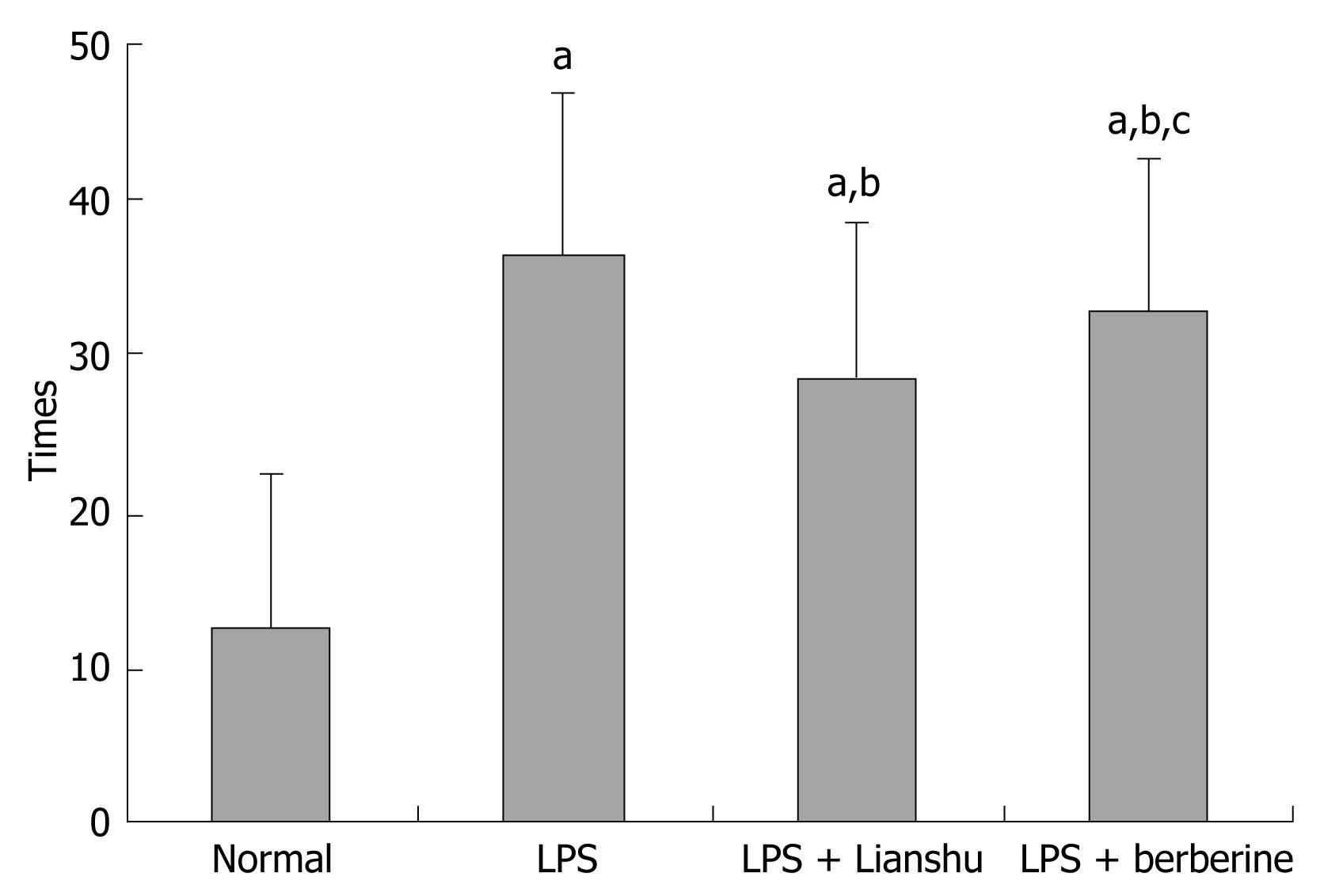
The frequency of diarrhea in LPS group, LPS + Lianshu group and LPS + berberine group was increased compared to normal group (36.70 ± 5.23, 28.50 ± 4.06, 32.70 ± 9.30 vs 12.40 ± 3.20 respectively, P < 0.01), significantly increased in LPS + Lianshu group and LPS + berberine group compared with normal group (P < 0.01), and decreased in LPS + Lianshu group compared with LPS + berberine group (P = 0.03) (Figure 1).
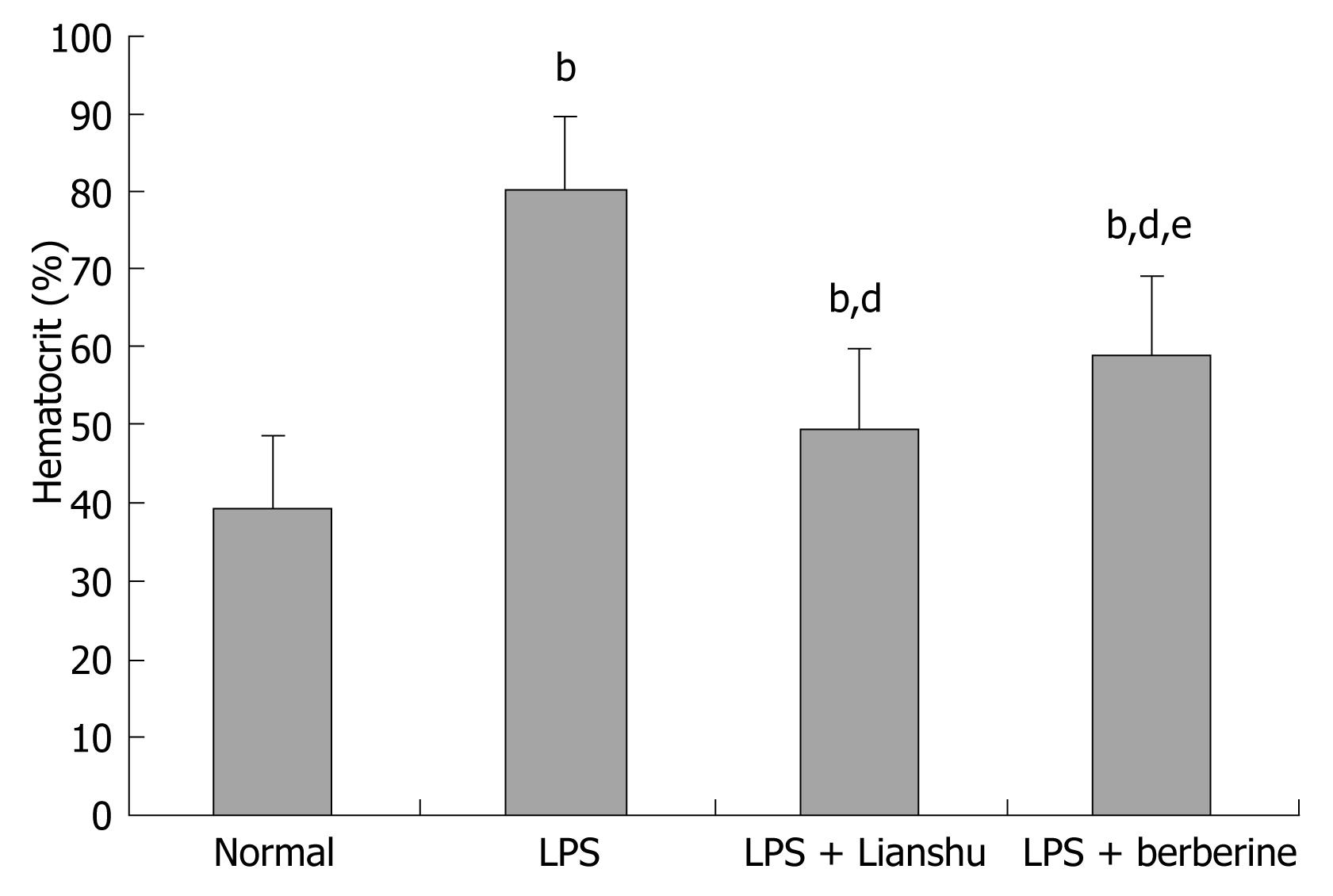
The levels of glucose, Na+, Cl-, and K+ were significantly lower in LPS + Lianshu group than in LPS + berberine group (140.35 ± 3.19 mmol/L vs 131.99 ± 4.86 mmol/L, 8.49 ± 1.84 mmol/L vs 6.54 ± 2.30 mmol/L, 106.29 ± 4.41 mmol/L vs 102.5 ± 1.39 mmol/L, 5.08 ± 0.66 mmol/L vs 4.32 ± 0.62 mmol/L respectively, P < 0.05), while the hematocrit level was lower in LPS + Lianshu group than in LPS + berberine group (0.50% ± 0.07% vs 0.59% ± 0.10%, P < 0.05, Table 1, Figure 2).
| Group | Sodium (mmol/L) | Glucose (mmol/L) | Chlorine (mmol/L) | Kalium (mmol/L) |
| Normal | 143.46 ± 3.34 | 8.37 ± 1.06 | 102.03 ± 2.82c | 5.37 ± 0.96 |
| LPS | 102.48 ± 3.67b | 4.99 ± 1.23b | 98.40 ± 1.42a | 3.38 ± 0.29b |
| LPS + Lianshu | 140.35 ± 3.19d | 8.49 ± 1.84d | 106.29 ± 4.41bd | 5.08 ± 0.66d |
| LPS + berberine | 131.99 ± 4.86bdf | 6.54 ± 2.30ae | 102.5 ± 1.39de | 4.32 ± 0.62bde |
The plasma NO and DAO levels were higher in LPS group than in normal group (P < 0.01) and lower in LPS + Lianshu group than in LPS + berberine group (P < 0.05, Table 2).
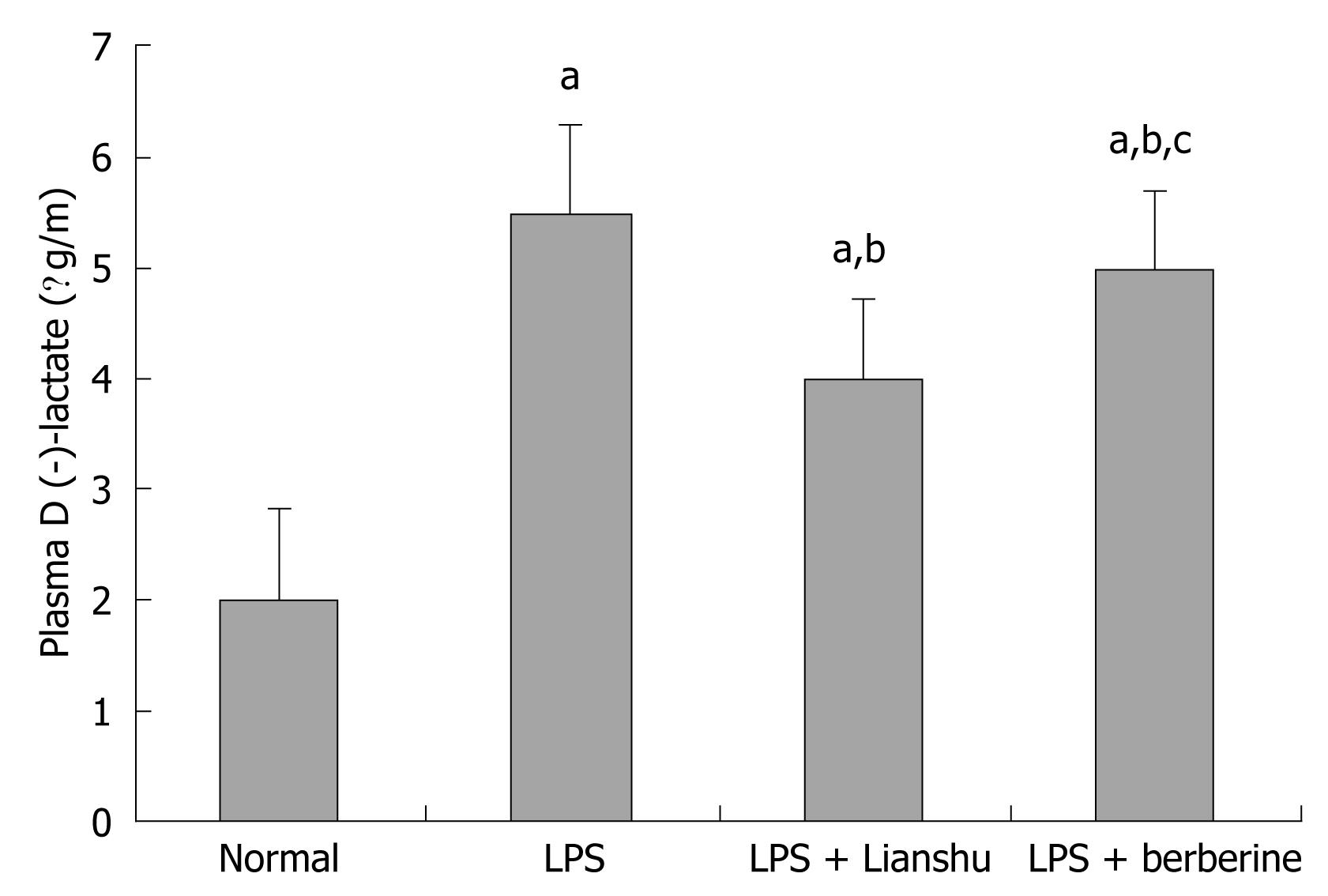
Plasma D (-)-lactate level was higher in LPS group, LPS + Lianshu group and LPS + berberine group than in normal group (5.36 ± 0.85, 4.00 ± 0.54, 4.88 ± 0.77 vs 2.01 ± 0.32 respectively, P < 0.01), and lower in LPS + Lianshu group, LPS + berberine group than in LPS group and LPS + berberine group (P < 0.01, Figure 3).
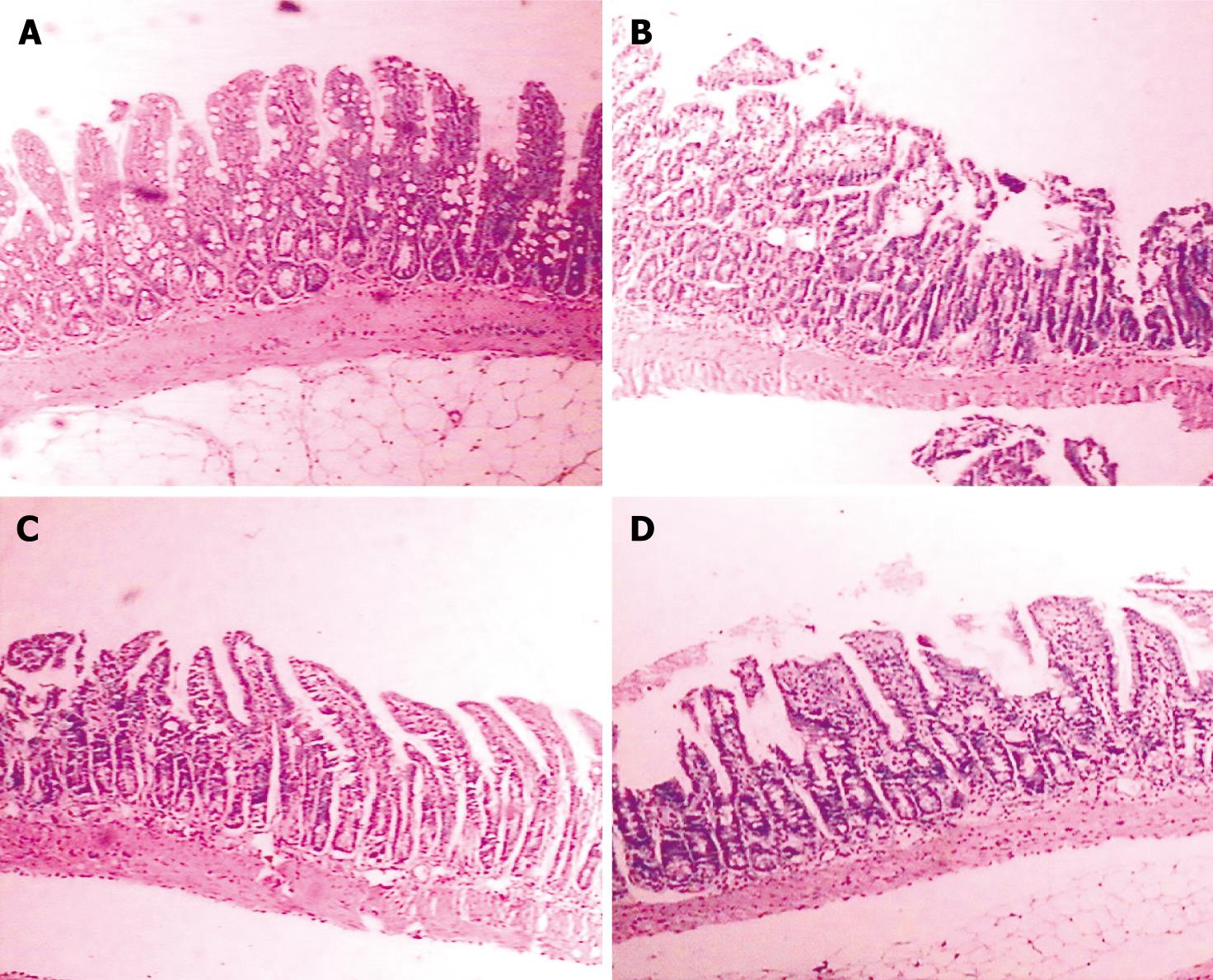
The villi of intestinal mucosa maintained their integrity and the shape of epithelial cells remained unchanged in the normal group. The villi were destroyed in the LPS group. Intestinal mucosal atrophy and inflammatory reaction were detectable in the other groups (Figure 4).
IgA+ cells, round or elliptical in shape, were distributed in the intestinal villi, lamina propria and intestinal glands. The endochylema was stained brown (Figure 5).
The number of IgA+ cells and amount of SIgA in intestinal fluid were less in LPS group than in normal group (P < 0.01) and higher in LPS + Lianshu group than in LPS + berberine group (P = 0.05, Table 3).
The temperature of rats in yeast group and sodium chloride group was similar 1 h after treatment with Lianshu preparation and higher in yeast group than in sodium chloride group 2-5 h after treatment with Lianshu preparation (P < 0.05). The temperature was not significantly changed in yeast + Lianshu group compared to yeast group 1 h or 2 h after treatment with Lianshu preparation. The temperature in yeast + Lianshu group decreased 3 h after treatment with Lianshu preparation (P = 0.025), and was almost identical to that in the normal group 4 h after treatment with Lianshu preparation. The temperature was lower in yeast + acetaminophen group than in the sodium chloride group 2 h after treatment with Lianshu preparation. Yeast + acetaminophen exhibited their effect 1 h earlier than yeast + Lianshu preparation (Table 4).
| Groups | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h |
| Normal | 0.58 ± 0.28 | 0.24 ± 0.71 | 0.20 ± 0.83 | 0.47 ± 0.30 | 0.17 ± 0.51 | 0.27 ± 0.65 |
| Yeast | 0.14 ± 0.94 | 0.75 ± 0.28b | 1.06 ± 0.28b | 1.16 ± 0.39b | 1.69 ± 0.43b | 1.46 ± 0.59b |
| Yeast + Lianshu | 0.30 ± 0.38 | 0.58 ± 0.38 | 0.44 ± 0.56c | 0.42 ± 0.29d | 1.01 ± 0.43c | 0.50 ± 0.44d |
| Yeast + acetaminophen | 0.11 ± 0.73 | 0.21 ± 0.39c | 0.37 ± 0.53c | 0.66 ± 0.50d | 0.74 ± 0.97ad | 0.53 ± 0.98d |
The rate of small intestinal peristalsis for mice after treatment with Lianshu preparation and atropine was different from that in mice after treatment with sodium chloride (55.20% ± 10.16% and 39.96% ± 8.57% vs 65.10% ± 10.93% respectively, P < 0.05, Figure 6).
A group of Japanese workers reported that parenteral LPS can lead to proliferation of gastrointestinal luminal bacteria in mice[4]. Subsequent work established that this enhancement of bacterial growth is secondary to fluid exudation, since the growth of bacteria could be suppressed by enteral unabsorbable antibiotics like neomycin[5]. Subcutaneous LPS from Gram-negative bacteria induces intestinal diarrhea characterized by destruction of the intestinal mucosa barricade[6–9].
In the present study, the frequency of diarrhea and gastrointestinal transit (GIT) were detected, and the levels of NO, DAO and D (-)-lactate were measured in mice after treatment with intraperitoneal LPS. Lianshu preparation could effectively prevent diarrhea by inhibiting enterokinesia. Generally, NO can protect and repair gastrointestinal mucosa. Endogenous NO has therapeutic effects on hypoxia, inflammation and damage. However, excess NO is probably one of the most im-portant mediators that induce blood poisoning, septic shock and multi-organ dysfunction[10]. When stimulated by LPS, NO is excessively expressed and induces multi-organ functional lesions in stomach and intestine[11–13]. In this study, Lianshu preparation prevented enteritis necroticans by inhibiting the production of excess NO. DAO is an ideal index of intestinal mucosal structure and function. The activity of DAO is closely related with villi, nucleic acid and protein synthesis in mucosal cells. When intestinal mucosal epidermis is damaged, DAO is released into blood, indicating that DAO in blood reflects the destruction of the intestinal mucosal epithelial cell layer and intestinal mucosa barricade[1415]. In our study, DAO was localized mostly in the epithelial cell layer of mucous membrane as previously described[16], and Lianshu preparation could effectively decrease DAO induced by LPS. However, its mechanism of action remains unclear. In addition, the function of the intestinal mucosa barricade could also be evaluated by measuring D (-)-lactate in plasma[17–19]. D (-)-lactate is a product of bacterial metabolism and schizolysis. However, mammals cannot produce D (-)-lactate[20]. D (-)-lactate cumulation in plasma reflects membrane permeability and barrier function of the intestinal mucosa[2122]. In our study, Lianshu preparation could reduce D (-)-lactate in plasma (P < 0.01) by protecting the barrier function of the intestinal mucosal and suppressing the overgrowth of harmful bacteria in the intestinal tract.
IgA is the main immunoglobulin in the mucosal immune system[23]. Polymeric IgA antibodies produced by plasma cells in the lamina propria of the intestinal tract bind to polymeric immunoglobulin receptors at the base of the epithelium. The complex of IgA and polymeric immunoglobulin receptors undergoes endocytosis and vesicular transport to the apical surface of enterocytes, and is secreted into the lumen. Generally, plasma cells are derived from B cells. It was reported that B cells are able to switch from IgM expression to IgA expression by Th2 cytokine activity in Peyer’s patches of the lamina propria and mesenteric lymph nodes, and then return to lamina propria of the intestinal tract via systemic circulation[24–26]. LPS, a mouse B-cell mitogen, induces B cell multiplication[26] and differentiation into plasma cells. LPS also activates macrophages to promote the production of cytokines such as interleukin (IL)-1, IL-6 and nitric oxide, which might regulate immunoglobulin production. Therefore IgA inhibits diarrhea caused by viruses[27–29]. Moreover, LPS might activate IgA production in the intestine[30]. However, in our study, the number of IgA+ cells and SIgA in the intestinal fluid was less in the LPS group than in normal group, and greater in the LPS + Lianshu group than in the LPS group, suggesting that high dose LPS leads to severe destruction of intestinal mucosa.
Infectious diarrhea is often caused by Gram-negative bacteria such as Escherichia coli. These organisms contain lipopolysaccharide (LPS). It is the important task of modern medicines to control infectious diarrhea. Antibiotics developed in the 1940s have saved millions of people’s lives. However, because of the widespread and inappropriate use of antibiotics, some bacterial strains become antibiotic-resistant. Folk herbal drugs have been studied for the treatment of acute diarrhea.
The method to treat diarrhea is mainly to kill bacteria or viruses. Lianshu preparation could destroy bacteria, prevent diarrhea, and protect the intestinal mucosa.
Lianshu preparation, comprised of non-antibiotic botanic components, has been used in the treatment of infectious diarrhea with a satisfactory effect. Lianshu preparation could effectively prevent diarrhea, relieve electrolyte disturbances and protect immune barriers. Its effect is better than berberine. In addition, Lianshu preparation has counteractive effects on pyrexia.
Lianshu preparation can be used as a natural medicine in treatment of infectious diarrhea with pyrexia. Chinese herbal drugs are effective against both chronic and acute diseases.
The authors confirmed, in their study, that Lianshu preparation was effective against LPS-induced diarrhea, its effect was better than berberine, suggesting that it can be used as a natural medicine in treatment of infectious diarrhea.
| 1. | Chittrakarn S, Sawangjaroen K, Prasettho S, Janchawee B, Keawpradub N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. J Ethnopharmacol. 2008;116:173-178. |
| 2. | Hu Sen, Duan ML, Xia B, Li JY, Chen TX, Zhang SW. Effects of Tongfu granules on small intestinal mucosal hemoperfusion and permeability during gut ischemia/reperfusion injury in dogs. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2006;6:331-334. |
| 3. | Brandt RB, Siegel SA, Waters MG, Bloch MH. Spectrophotometric assay for D-(-)-lactate in plasma. Anal Biochem. 1980;102:39-46. |
| 4. | Creydt VP, Nuñez P, Zotta E, Ibarra C. [Cytotoxic effect of Shiga toxin type 2 and its B subunit on human renal tubular epithelial cell cultures]. Medicina (B Aires). 2005;65:147-50. |
| 5. | Niyogi SK. Shigellosis. J Microbiol. 2005;43:133-143. |
| 6. | Nöldner M, Schötz K. Inhibition of lipopolysaccharid-induced sickness behavior by a dry extract from the roots of Pelargonium sidoides (EPs 7630) in mice. Phytomedicine. 2007;14 Suppl 6:27-31. |
| 7. | Moriez R, Salvador-Cartier C, Theodorou V, Fioramonti J, Eutamene H, Bueno L. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071-1079. |
| 8. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. |
| 9. | Liu J, Wang ZT, Ji LL, Ge BX. Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol Cell Biochem. 2007;298:49-57. |
| 10. | Borghan MA, Mori Y, El-Mahmoudy AB, Ito N, Sugiyama M, Takewaki T, Minamoto N. Induction of nitric oxide synthase by rotavirus enterotoxin NSP4: implication for rotavirus pathogenicity. J Gen Virol. 2007;88:2064-2072. |
| 11. | Takahashi J, Sekine T, Nishishiro M, Arai A, Wakabayashi H, Kurihara T, Hashimoto K, Satoh K, Motohashi N, Sakagami H. Inhibition of NO production in LPS-stimulated mouse macrophage-like cells by trihaloacetylazulene derivatives. Anticancer Res. 2008;28:171-178. |
| 12. | Kawanishi N, Tanaka Y, Kato Y, Shiva D, Yano H. Lipopolysaccharide-induced monocyte chemotactic protein-1 is enhanced by suppression of nitric oxide production, which depends on poor CD14 expression on the surface of skeletal muscle. Cell Biochem Funct. 2008;26:486-492. |
| 13. | Talukder MJ, Harada E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can J Physiol Pharmacol. 2007;85:200-208. |
| 14. | Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185-1196. |
| 15. | Toporowska-Kowalska E, Wasowska-Królikowska K, Bodalski J, Fogel A, Kozłowski W. Diamine oxidase plasma activity and jejunal mucosa integrity in children with protracted diarrhoea. Rocz Akad Med Bialymst. 1995;40:499-503. |
| 16. | Usami M, Ohata A, Kishimoto K, Ohmae K, Aoyama M, Miyoshi M, Fueda Y. Phospholipid fatty acid composition and diamine oxidase activity of intestinal mucosa from rats treated with irinotecan hydrochloride (CPT-11) under vegetable oil-enriched diets: comparison between perilla oil and corn oil. JPEN J Parenter Enteral Nutr. 2006;30:124-132. |
| 17. | Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg. 2003;237:101-109. |
| 18. | Nakayama M, Yajima M, Hatano S, Yajima T, Kuwata T. Intestinal adherent bacteria and bacterial translocation in breast-fed and formula-fed rats in relation to susceptibility to infection. Pediatr Res. 2003;54:364-371. |
| 19. | Cağlayan F, Cakmak M, Cağlayan O, Cavuşoglu T. Plasma D-lactate levels in diagnosis of appendicitis. J Invest Surg. 2003;16:233-237. |
| 20. | Ewaschuk JB, Naylor JM, Zello GA. D-lactate in human and ruminant metabolism. J Nutr. 2005;135:1619-1625. |
| 21. | Lorenz I, Vogt S. Investigations on the association of D-lactate blood concentrations with the outcome of therapy of acidosis, and with posture and demeanour in young calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med. 2006;53:490-494. |
| 22. | Ewaschuk JB, Naylor JM, Palmer R, Whiting SJ, Zello GA. D-lactate production and excretion in diarrheic calves. J Vet Intern Med. 2004;18:744-747. |
| 23. | Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153-245. |
| 24. | McIntyre TM, Strober W. Gut-associated lymphoid tissue: regulation of IgA B cell development. Mucosal Immunology. Academic Press: San Diego 1999; 319-356. |
| 25. | Phillips-Quaglita JM, Lamm ME. Lymphocyte homing to mucosal effector Sites. Academic Press: San Diego 1994; 225-239. |
| 26. | Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199-205. |
| 27. | Reimerink JH, Boshuizen JA, Einerhand AW, Duizer E, van Amerongen G, Schmidt N, Koopmans MP. Systemic immune response after rotavirus inoculation of neonatal mice depends on source and level of purification of the virus: implications for the use of heterologous vaccine candidates. J Gen Virol. 2007;88:604-612. |
| 28. | Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122:175-181. |
| 29. | Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain). J Virol. 2007;81:9183-9192. |
| 30. | Hisajima T, Kojima Y, Yamaguchi A, Goris RC, Funakoshi K. Morphological analysis of the relation between immunoglobulin A production in the small intestine and the enteric nervous system. Neurosci Lett. 2005;381:242-246. |