CASE REPORT
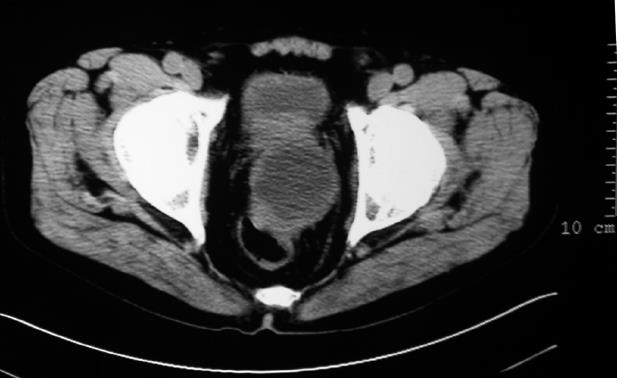
A 72-year-old man presented at the hospital with a 6-mo history of intermittent melena, mild lower abdominal discomfort and intussusception. At the time of admission, his complete blood count (CBC) revealed anemia with a low hemoglobin concentration of 75 g/L. An abdominal ultrasound and a computerized tomography (CT) scan of the abdomen and pelvis revealed a solitary irregular and low-density mass of 7 cm × 6 cm in the rectum, extending from the anterior rectal wall into the prostate. The urinary bladder was compressed anteriorly and tightly attached. X-ray showed no intra-abdominal lymphadenopathy or liver metastases (Figure 1) or any lung abnormality. Then, colonscopy and ultrasound endoscopy revealed a submucous mass in the rectum without any visible mucosal abnormality or intraluminal blood. The mass was localized at about 6.5 cm from the anal verge. However, the histological examination of the rectal biopsy, performed three times at other two hospitals, revealed a normal tissue with a mild infiltrate of inflammatory cells. A provisional diagnosis of rectal GIST was suspected by the CT scan.
Figure 1 CT scan image at pelvic showing a large low-density lesion arising from the rectum.
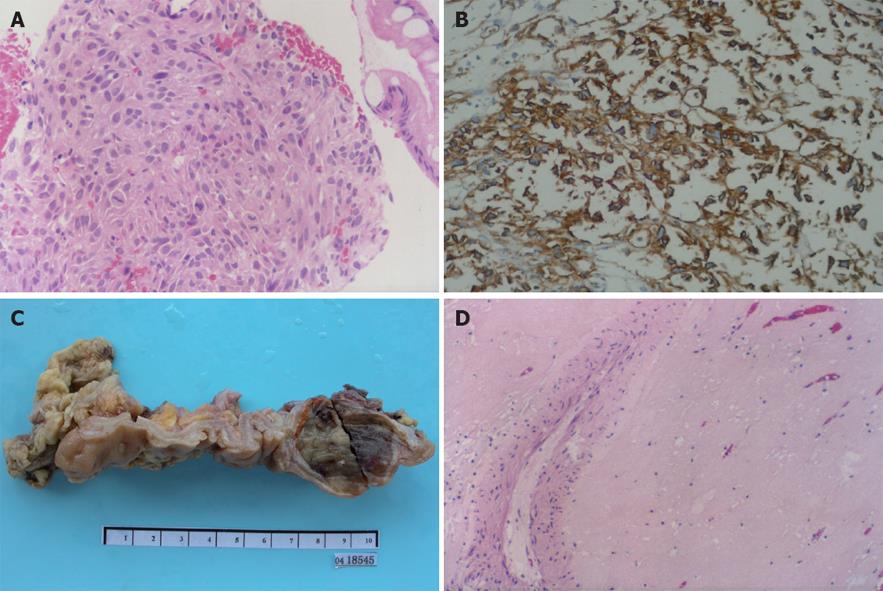
The patient was admitted at the hospital and transfused with 3 units of packed red blood cells. Considering the patient’s age, the tumor localization and size and its relationship with the neighbouring organs, a definitive histopathological diagnosis was mandatory in order to adopt appropriate treatment. To do so, a rectal subserosa fine-needle deep biopsy was successfully obtained under the guidance of ultrasound endoscopy. Histologically, spindle cells were detected in one of five sections of the tissue biopsy. The cells exhibited moderate to severe atypia and active mitoses, with a total of six mitotic figures in two high-power fields (Figure 2A). Immunohistochemistry showed strong and diffuse staining for CD117 (Figure 2B) and CD34, but proved negative for all other differentiation markers such as α-SMA, MSA, desmin, S-100 protein, and HMB45. Based on the histopathological exam, the final diagnosis of a high grade malignant GIST was made. There was in sufficient tumor tissue to examine the genetic mutations for the c-kit and PDGFRα genes.
Figure 2 Different images of the rectal tumor.
A: Histopathological microphotograph of biopsy tissue showing spindle-shaped cells with obvious atypia and active mitoses (HE, × 200); B: Envision immunohistochemical stained tumor cells showing strong and diffuse positive staining for CD117 (HE, × 200); C: Macroscopic image of the resected tissue showing a submucosal tumor grayish and uniformly soft tissue texture, measuring 4 cm × 3.5 cm × 3 cm in size; D: Histological examination of the resected tissue showing no residual tumor cells, except for blood vessels and scattered lymphocytes (HE, × 200).
Because of the size and the localization of the lesion, both surgical intervention and the possibility of IM neoadjuvant were recommended to the patient and his family members. All declined radical excision and consented to IM neoadjuvant. The patient received IM therapy with a single dose of 400 mg administered daily for one and a half months, and was followed up by CT scans of the chest, abdomen and pelvis to monitor the outcome. The tumor dramatically shrunk to 5 cm after one month, and then developed complete cystic changes and sharp demarcation with respect to neighbouring organs after another half a month, when an exploratory laparotomy was carried out, followed by a segmental colorectum resection. The surgery was performed more easily than we would have anticipated for a similar case without IM therapy. Due to a delayed postoperative recovery, the patient was discharged one week later. Visual observation of the resected tissue found a grayish tumor of 4 cm × 3.5 cm × 3 cm in the muscularis propria (Figure 2C). Histopathology examination revealed that the tumor had completely responded to IM neoadjuvant since there was no viable tumour cells found in the extirpated specimen (Figure 2D). Satisfied with the histopathologic results, the patient declined continuous IM adjuvant therapy. He has been followed up and his disease free survival is currently at 57 mo after the combined therapy: no signs of recurrence or relapse of the tumor have occurred so far.
DISCUSSION
GISTs are the most common mesenchymal tumors of the gastrointestinal tract, most frequently found in the stomach (60%) and the small intestine (30%). The incidence of the rectal GIST is low (4%)[34]. Although abdominoperineal resections of large sized GISTs are not sufficient to prevent the highly frequent recurrence of the tumor[5], surgical treatments, including radical resection and local excision, remain the main treatment for primary rectal GISTs. However, for rectal GISTs, its rarity makes it difficult to assess the role of the extension of the surgical resection of the tumor.
IM selectively inhibits the enzymatic activity of tyrosine kinases, such as ABL, PDGFR, and KIT. Its development was a landmark event in the history of cancer therapy. With IM adjuvant treatment, approximately 60%-80% of patients with recurrent or metastatic GISTs achieve a partial response or stable disease; but, complete response induced by IM is rare. The lack of complete response in many patients with partial response may be due to the drug resistance developed with a prolonged treatment. In fact, studies showed that half of the patients no longer respond to IM after approximately two years of continuous treatment[6–8], and the likelihood of developing IM resistance is proportional to the size of residual viable GIST. Therefore, alternative and more efficient therapies for primary GISTs are required in the IM era. Currently, a potential paradigm shift in the management of GISTs has been proposed and explored, and whether IM neoadjuvant can improve outcome has been broadly evaluated.
Until now, the evaluation criteria for IM neoadjuvant therapy have not been well defined. GISTs comprise a wide range of biological behaviors, including benign, borderline and malignant variants[9]. Surgery remains the treatment of choice for benign and borderline GISTs. Preoperative diagnosis is the most critical criterion for selecting the appropriate therapy. Most alimentary tract diseases can be initially diagnosed by endoscopy, imaging and other methods; pathologic examination of tissue specimens is the most valuable final confirmation of the diagnosis. GISTs are submucous tumors with the surface covered with normal mucosa. A common problem for the diagnosis of GISTs is that conventional endoscopic biopsies using forceps are often unsuccessful in sampling submucosal mass, because they are too superficial and only obtain biopsy samples of the mucosal tissue. EUS-guided aspiration or core biopsy for submucosal tumors had a success rate of 60%-70% in the earlier studies, but this rate is now gradually reaching 80%-90%, which is significantly higher than that of a simple endoscopic biopsy[10–12]. The usefulness of biopsy in diagnosis can be enhanced by immunohistochemical staining on aspirates or tissues for tumor-specific markers such as CD117 and CD34, which are crucial for a diagnosis of GISTs[13]. Severe nuclear atypia and active mitotic figures indicating malignancy can help the decision in favor of a neoadjuvant therapy. For this reason, four biopsies were obtained from our patient to confirm the diagnosis before starting the therapy.
IM neoadjuvant therapy for primary GISTs has been reported[14–18]. Among the reports, there are five gastric and one intestinal GISTs, where the tumor mass size made it unresectable, two primary rectal GISTs[1920], and one recurrent rectal GIST where the abdominoperineal resection of the rectum could not be done[21]. Sparing surgery with preservation of the anal sphincter was possible in all of the three rectal GIST cases reported previously as well as in our case. In the case reported by Lo et al[19], residual tumor cells were found 2.5 mo after IM therapy. In another case, reported by Shah et al[20], no data are available concerning any residual tumor 6.5 mo after IM neoadjuvant therapy. One recurrent rectal case, reported by Salazar et al[21], showed complete histopathological response 12 mo after IM therapy. In our case, the patient achieved complete response after only one and half months of IM neoadjuvant therapy. To our knowledge, this is the shortest time to achieve complete response by IM therapy. Since the patient declined postoperatively IM adjuvant treatment, he was then closely followed up. The patient has been asymptomatic and disease-free since then, i.e. 57 mo after the combined treatment. It is reasonable to speculate that the 57 mo disease-free survival from the combined treatment is largely due to the preoperative IM therapy.
Recently, Andtbacka et al[22] compared the outcomes of preoperative IM therapy combined with surgery with locally advanced and metastatic GISTs among 46 patients, including 11 with locally advanced primary tumors and 35 with metastatic tumors. Complete surgical resection after IM neoadjuvant was accomplished in all of the advanced primary tumors, but only 31.4% (11/35) of the metastatic tumors. Based on these results, IM neoadjuvant combined with surgery can be considered as more effective for patients with primary GISTs.
Based on our prior experience and the findings from this case, we believe that the approach of IM neoadjuvant treatment combined with surgery should offer the patient a longer event-free and overall survival.