Published online Apr 21, 2009. doi: 10.3748/wjg.15.1869
Revised: March 14, 2009
Accepted: March 21, 2009
Published online: April 21, 2009
AIM: To investigate the clinical implications of lipid deposition in the pancreas (fatty pancreas).
METHODS: The subjects of this study were 293 patients who had undergone abdominal computed tomography (CT) and sonography. Fatty pancreas was diagnosed by sonographic findings and subdivided into mild, moderate, and severe fatty pancreas groups comparing to the retroperitoneal fat echogenicity.
RESULTS: Fatty pancreas was associated with higher levels for visceral fat, waist circumference, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, triglyceride, high density lipoprotein, free fatty acid, γ-GTP, insulin, and the homeostasis model assessment of insulin resistance (HOMA-IR) than the control group (P < 0.05). HOMA-IR, visceral fat, triglyceride, and ALT also tended to increase with the degree of fat deposition in the pancreas on sonography. In a multivariate logistic regression analysis, HOMA-IR, visceral fat, and ALT level were independently related to fatty pancreas after adjustment for age, body mass index, and lipid profile. The incidence of metabolic syndrome in the fatty pancreas group was significantly higher than in the control group, and the numbers of metabolic syndrome parameters were significantly higher in the fatty pancreas group (P < 0.05).
CONCLUSION: Sonographic fatty pancrease showed higher insulin resistance, visceral fat area, triglyceride, and ALT levels than normal pancreases. Fatty pancreas also showed a strong correlation with metabolic syndrome.
- Citation: Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, Son BK, Kim SH, Jo YJ, Park YS, Kim YS. Clinical implications of fatty pancreas: Correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol 2009; 15(15): 1869-1875
- URL: https://www.wjgnet.com/1007-9327/full/v15/i15/1869.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1869
It has been reported that increases in triglyceride and free fatty acids causes ectopic fat deposition in the liver, heart, muscles, and pancreas. This is called steatosis and is known to be related to obesity and/or insulin resistance[1]. In particular, steatosis in the liver (or fatty liver) refers to fat deposition in hepatocytes, and its pathophysiology, diagnostic criteria, clinical implications are already well known. However, few studies have been done on lipid deposition in the pancreas (fatty pancreas) and its clinical implications.
A recent animal study reported that lipid deposition in pancreas islet cells due to a high fat/high glucose diet could damage pancreatic beta cells and make them hyperglycemic[1]. However, another study reported that there was no histological evidence of the existence of steatosis in the human pancreas and no reports have clearly demonstrated that the increase of pancreas echogenicity implied steatosis of the pancreas or fat deposition[2]. Autopsy studies reported pathologic findings of pancreatic steatosis in interlobular septa rather than in pancreas acinar cells[3–5], and fat deposition related to aging[67]. According to an animal study, pancreatic steatosis caused anomalies of pancreas islet cells leading to hyperglycemia[8–10]. Some authors suggested that pancreas islet cell damage that occurs in pancreatic steatosis is accompanied by hyperlipidemia and this plays an important part in the generation of type 2 diabetes[11]. Gullo et al[12] suggested that hypercholesterolemia causes fat deposition in the pancreas and this change is related to hyperamylasemia. In this study we investigated the clinical implications of fatty pancreas and the correlation between insulin resistance and metabolic syndrome.
The subjects of this study were adults who visited an obesity clinic and for whom doctors performed physical examinations and took their medical histories. Those who had diabetes, pancreatic disease, thyroid disease, renal disease, liver disease, and who drank alcohol over 40 g daily for male and 20 g daily for female were excluded from the subjects. Among them, 293 people who underwent abdomen sonography and/or fat measurement CT for evaluation of abdominal fat distribution were chosen as the subjects. Daily alcohol intake, past history, and other general characteristics of the subjects were collected through questionnaires.
We measured the height and body weight of subjects in light clothing to calculate the Body Mass Index (BMI = kg/m2). For waist circumference, we used a tape measure and measured in the middle part between the lowest rib and the iliac crest of the pelvis, horizontal to the ground with the subject in an upright position. Blood samples were test for liver functions, fasting glucose, HbA1c, insulin, total cholesterol, triglyceride, high density lipoprotein, and low density lipoprotein, after fasting for 12 h.
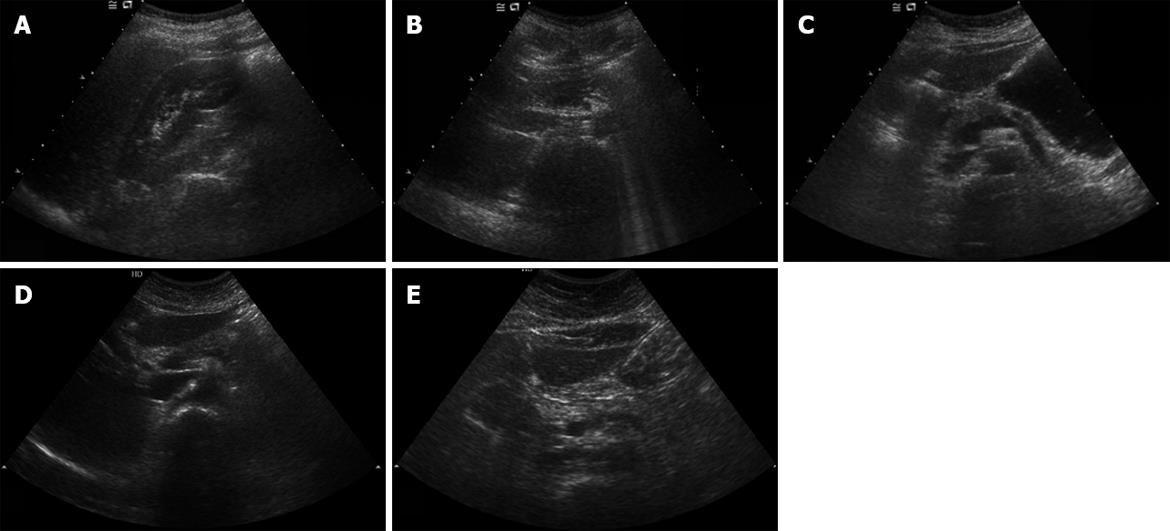
All subjects received tests with the same sonograph (Envisor HD, Phillips, Bothell USA) using a convex 5-2 MHz transducer by one radiologist to minimize biases by different testers. The increase of echogenicity of the pancreatic body over the kidney echogenicity was classified as fatty pancreas, and in other cases as non-fatty pancreas. As the pancreas could not be compared with the kidney in the same window, the radiologist compared pancreatic echogenicity with kidney echogenicity using the difference between liver and kidney echogenicity and liver and pancreas echogenicity. Fatty pancreas was subdivided into four stages: control group (non-fatty pancreas), where the pancreas echogenicity was similar to the kidney parenchymal echogenicity; light fatty pancreas, where the pancreas echogenicity was higher than the kidney echogenicity, but very much lower than the retroperitoneal fat echogenicity; severely fatty pancreas, where the pancreas echogenicity was higher than the kidney echogenicity, but a little lower than the retroperitoneal fat echogenicity; and highly fatty pancreas, where the pancreas echogenicity was similar to the retroperitoneal fat echogenicity.
Abdomen visceral fat, subcutaneous fat, total fat, and thigh muscle fat areas were measured. A 16-channel multiplex abdominal CT system (General Electric Medical Systems, Milwaukee, USA) was used and measurements were made at the location of the umbilicus and middle part of the thigh. The embedded computer was used for calculations. Hounsfield units (HU) were measured at five different parts of the pancreas (head, neck, body, tail, and uncinate process) and three different parts of the spleen. The differences between the mean values of them were determined. If this difference was -5 or lower, it was classified into the fatty pancreas group and the rest were classified into the non-fatty pancreas group.
Insulin concentration was measured using a chemiluminescent immunoassay (Immulite 2000, Diagnostic Products Corp., Los Angeles, CA; CV, < 7%). The measure of insulin resistance was obtained using the HOMA-IR (Homeostasis model assessment-insulin resistance), and the calculation formula shown below: HOMA-IR: [Fasting blood sugar (mmol/L) × Fasting insulin (&mgr;U/mL)/22.5][13].
The criteria for metabolic syndrome diagnosis followed the NCEP-Adult Treatment Panel III (ATP III), and the visceral obesity was defined by substituting it with the standard waist circumference in the Asia-Pacific Region. Diagnostic criteria were defined as when three or more items of the following were met: visceral obesity (waist circumference ≥ 90 cm for males, or waist circumference ≥ 80 cm for females), increased triglyceride (≥ 150 mg/dL), decreased HDL (< 40 mg/dL for males, < 50 mg/dL for females), hypertension (≥ 130/85 mmHg), and fasting glucose (≥ 110 mg/dL).
For statistical analysis, the SPSS for Windows (version 11.0; SPSS, Chicago, Ill) was used. The Student’s t-test was used for comparison between the two groups according to the existence or non-existence of fatty pancreas; the χ2 test for relationship between fatty pancreas and metabolic syndrome; the multiple logistic analysis was used for analysis of independent correlation factors related to fatty pancreas; and the ANOVA test was used to compare the four groups according to the severity of fatty pancreas.
The mean age of 293 subjects was 44.9 ± 9.5 years. There were 133 males (45.4%) and 160 females (54.6%). Among them, 180 (61.4%) were diagnosed as having fatty pancreas from the abdomen sonography; 93 males (51.7%) and 87 females (48.3%). The subjects were divided into the fatty pancreas group and the non-fatty pancreas group for comparison of clinical characteristics. In the fatty pancreas group to compared non-fatty pancreas group, the mean body mass index (26.5 ± 3.1 kg/m2vs 24.4 ± 3.2 kg/m2, P < 0.001), waist circumference (88.9 ± 8.5 vs 82.1 ± 9.3, P < 0.001), and visceral fat (10767 ± 4260 vs 7462 ± 3244, P < 0.001) were statistically higher. The aspartate aminotransferase (AST) (30.2 ± 20.7 vs 23.8 ± 11.1, P = 0.001), alanine aminotransferase (ALT) (40.3 ± 32.2 vs 25.0 ± 23.0, P < 0.001), total cholesterol (205.2 ± 35.2 vs 192.9 ± 36.4, P = 0.005), triglyceride (159.8 ± 92.5 vs 119.6 ± 70.3, P < 0.001), high density lipoprotein cholesterol (47.7 ± 10.6 vs 51.9 ± 10.1, P = 0.001), γ-glutamyl transpeptidase (γ-GGT) (46.5 ± 43.0 vs 29.9 ± 29.9, P < 0.001), fasting insulin concentration (6.8 ± 3.6 vs 5.4 ± 2.1, P < 0.001), and homeostasis model assessment of insulin resistance (HOMA-IR) (3.2 ± 2.1 vs 2.3 ± 1.0, P < 0.001), free fatty acid (767.9 ± 324.9 vs 639.3 ± 287.6, P = 0.001) also showed significant differences between the two groups (P < 0.05). However, age, fasting glucose, low-density lipoprotein, and cholesterol concentration did not show any differences (Table 1).
| Non-fatty pancreas (n = 113) | Fatty pancreas (n = 180) | P-value | |
| Age (yr) | 44.4 ± 9.7 | 45.4 ± 8.5 | NS |
| Sex (male/female) | 34/79 | 93/87 | < 0.001 |
| BMI (kg/m2) | 24.4 ± 3.2 | 26.5 ± 3.1 | < 0.001 |
| WC (cm) | 82.1 ± 9.3 | 88.9 ± 8.5 | < 0.001 |
| Visceral Fat(mm2) | 7462 ± 3244 | 10767 ± 4260 | < 0.001 |
| AST (IU/L) | 23.8 ± 11.1 | 30.2 ± 20.7 | 0.001 |
| ALT (IU/L) | 25.0 ± 23.0 | 40.3 ± 32.2 | < 0.001 |
| FBS (mg/dL) | 99.7 ± 24.0 | 102.9 ± 26.2 | NS |
| TC (mg/dL) | 192.9 ± 36.4 | 205.2 ± 35.2 | 0.005 |
| TG (mg/dL) | 119.6 ± 70.3 | 159.8 ± 92.5 | < 0.001 |
| LDL (mg/dL) | 118.6 ± 36.3 | 124.8 ± 27.4 | NS |
| HDL (mg/dL) | 51.9 ± 10.1 | 47.7 ± 10.6 | 0.001 |
| γ-GT (mg/dL) | 29.9 ± 29.9 | 46.5 ± 43.0 | < 0.001 |
| Fasting insulin (&mgr;U/mL) | 5.4 ± 2.1 | 6.8 ± 3.6 | < 0.001 |
| HOMR-IR | 2.3 ± 1.0 | 3.2 ± 2.1 | < 0.001 |
| FFA (mg/dL) | 639.3 ± 287.6 | 767.9 ± 324.9 | 0.001 |
To analyze the factors related to fatty pancreas, we conducted a multiple logistic analysis for variables, which were found to have significant relationships with fatty pancreas from the univariate analysis. The multiple logistic analysis was conducted by adding variables in three steps. In the first stage, Model I, age, sex, HOMA-IR, and fasting glucose were used as the independent variables and fatty pancreas as the dependent variable. In Model II, triglyceride and free fatty acid, which were already known to be related to fatty liver, were added to Model I. In Model III, abdomenal visceral fat, subcutaneous fat area, and thigh muscle fat area were added to Model II to evaluate the body fat distribution. The multiple logistic analysis found that HOMA-IR had a strong correlation with fatty pancreas, even after correction of age and sex, and this did not change even after correction for triglycerides, cholesterol and free fatty acid. However, after correction with the visceral fat area, the visceral fat showed the strongest correlation with fatty pancreas; but, HOMA-IR did not show a significant correlation (Table 2).
| Model | Odds ratio (95% CI) | P-value | |
| Model 1 | Age (yr) | 1.025 (0.999-1.051) | 0.059 |
| Sex (male/female) | 0.397 (1.474-4.311) | 0.001 | |
| Fasting glucose (mg/dL) | 0.998 (0.987-1.010) | 0.747 | |
| HOMA-IR | 2.319 (1.431-3.757) | 0.001 | |
| Model 2 | Model 1 | ||
| (HOMA-IR) | 1.761 (1.041-2.978) | 0.035 | |
| + | |||
| TC (mg/dL) | 1.005 (0.998-1.013) | 0.184 | |
| TG (mg/dL) | 1.004 (1.000-1.008) | 0.060 | |
| FFA (mg/dL) | 1.000 (0.999-1.001) | 0.409 | |
| Model 3 | Model 2 | ||
| (HOMA-IR) | 0.990 (0.533-1.839) | 0.973 | |
| + | |||
| BMI (kg/m2) | 1.137 (0.965-1.339) | 0.125 | |
| Visceral fat | 1.000 (1.000-1.000) | 0.006 | |
| Subcutaneous fat | 1.000 (1.000-1.000) | 0.932 | |
| Thigh fat | 1.000 (1.000-1.000) | 0.214 |
An analysis of 104 subjects who showed metabolic syndrome based on ATP III criteria found fatty pancreas in the sonography of 80 (76.9%) patients, which was a significantly higher proportion of the fatty pancreas group (P < 0.001). Furthermore, the number of metabolic syndrome parameters (waist circumference, HDL, triglyceride, fasting glucose, blood pressure) in the fatty pancreas group (2.3 ± 1.4) was statistically significantly higher than that of the non-fatty pancreas group (1.4 ± 1.2) (P < 0.001) (Table 3).
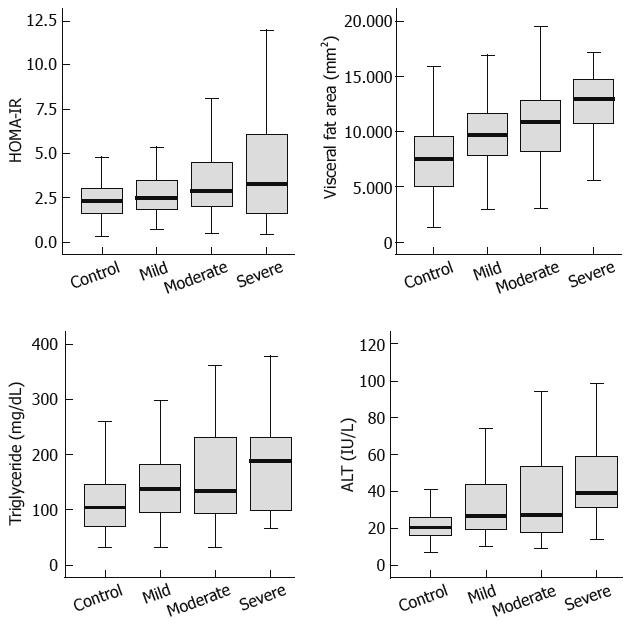
Among the 180 subjects who showed fatty pancreas, 90 patients were found to have mild fatty pancreas, 68 moderate fatty pancreas, and 22 severe fatty pancreas. The four groups, including the control group of non-fatty pancreas, were compared. We analyzed the correlation between HOMA-IR, visceral fat, triglyceride, and ALT, which were found to be related to fatty pancreas by multivariate multiple logistic analysis and the degree of fatty pancreas. HOMA-IR, visceral fat, triglyceride, and ALT tended to increase with the degree of fat deposition in the pancreas on sonography (Figure 1; Table 4).
| Ultrasonographic severity of fatty pancreas (γ) | P-value | |
| HOMA-IR | 0.250 | < 0.001 |
| Visceral fat | 0.396 | < 0.001 |
| TG (mg/dL) | 0.245 | < 0.001 |
| ALT (IU/L) | 0.276 | < 0.001 |
To determine correlations between metabolic parameters and fatty pancreas appearing on CT finding pancreas and those found by sonography, the difference between the average Hounsfield Units (HU) from mean pancreas HU to mean spleen HU was calculated. If the difference was -5 or lower the subjects were classified into the fatty pancreas group on CT finding, and others were classified into the non-fatty pancreas group. A comparison of metabolic syndrome factors and body measurement factors found no difference in visceral fat, lipid profile, and liver chemistry between the two groups, based on CT findings.
The total number of fatty pancreas or fatty liver patients was 184. Concurrence of fatty pancreas and fatty liver on sonography was found in 125 subjects (67.9%); fatty pancreas with normal liver was found in 55 (29.9%) and fatty liver without fatty pancreas was only found in four (2.2%) patients. In other words, among 180 fatty liver patients, 125 subjects also had fatty pancreases (96.8%), and the negative predictive value of fatty liver in normal pancreas was 96.4%
The pathophysiology and diagnostic criteria for steatosis in liver or fatty liver are already established. Pancreas echogenicity in abdomen sonography is known to be determined by peripancreatic fat deposition rather than pancreatic parenchymal deposition[6], and it was reported that this correlated with age and subcutaneous fat[14]. However, there have been few studies on lipid deposition in the pancreas and its clinical implications.
Sonography has been widely used as a tool for evaluation of fat deposition in the pancreas, and the pancreas echogenicity has been traditionally compared with liver echogenicity[714]. However, this does not seem to be a good method, because the liver is metabolically very active and its echogenicity exhibits high variance[1516]. The concurrence of fatty pancreas and fatty liver on sonography was very high in our data (67.9%). Therefore, liver echogenicity was not a good reference value for diagnosis of fatty pancreas. In contrast, spleen and kidney are known to be metabolically less variable than liver. Therefore, the authors compared the pancreas echogenicity with kidney parenchymal echogenicity, unlike prior studies. This seems to be a reasonable method because the kidney is metabolically more stable than the liver, although it is difficult to compare both the pancreas and the kidney in the same window.
We performed a multiple logistic regression analysis that corrected for age, sex, and serum lipid and found that insulin resistance was independently related to the existence of fatty pancreas in various models. Subjects with fatty pancreas showed strong association with frequency of metabolic syndrome, and fatty pancreas correlated with a number of the parameters of metabolic syndrome, including insulin resistance. However, after adjusting for factors related to body fat distribution, particularly visceral fat, the strong association with insulin resistance disappeared. The reason for this seems to be that visceral fat is a much stronger relational factor and influenced the relationship between fatty pancreas and insulin resistance. This suggests that fatty pancreas is a risk factor for metabolic syndrome or another manifestation of metabolic syndrome, such as the previously reported correlations of nonalcoholic fatty liver disease with obesity, insulin resistance, and metabolic syndrome[1718].
Furthermore, this study subdivided fatty pancreas by the degree of pancreas echogenicity, and compared it with HOMA-IR, visceral fat, triglyceride, and ALT. The correlation coefficients were 0.250, 0.396, 0.245, and 0.276, respectively (P < 0.001), demonstrating statistically significant correlation with fatty pancreas severity (Figure 2). A comparative analysis with various other factors is needed with a greater number of subjects.
This study found that HOMA-IR, an insulin resistance marker, had an independently significant correlation with fatty pancreas; but, there were no differences in fasting blood sugar between the two groups. The reason for this seems to be that diabetes was excluded from the selection conditions for subjects.
To compare the echogenicity on sonography with objective Hounsfield units on CT, this study conducted mean pancreas and spleen HU, and analyzed the clinical meaning of fatty pancreas using by CT imaging. However, unlike sonography, no statistical difference in clinical and biochemical parameters could be found. It seems that fat deposition in the pancreas shows different patterns from that in the liver. There is a report that fat deposition was markedly increased in pancreas islet cells of people aged over 60 years[19], and an animal study demonstrated lipid deposition in pancreas islet cells induced by a high fat/high sucrose diet[1]. On the other hand, another study claimed that fat deposition in the human pancreas does not occur in the parenchymal cells of the pancreas, but is only limited to interstitial stroma[20]. Some researchers insist that steatosis in the pancreas or fat deposition in pancreatic cells does not exist in humans[12]. However, according to some studies that contained pathological observations on the human pancreas[3–5], fat deposition in the human pancreas appears to occur mainly in interlobular septa, rather than in cells. However, it is difficult to arrive at clear conclusions due to the difficulty of tissue collection from the pancreas, which limits histological proof. The results of the subgroup analysis, which investigated the relationship of degree of fatty pancreas with CT, seems to be due to such characteristics of fat deposition in the pancreas. In other words, because pancreas fat deposition mainly occurs in interlobular septa, the CT images of pancreas show severe non-homogenous patterns with big differences in Hounsfield Units depending on the part measured. Consequently, for CT judgment of pancreas fat deposition it is more appropriate to evaluate the degree of irregular lobulated contour by fat deposition in interlobular septa rather than by Hounsfield Units[62021]. Therefore, sonography seems to be the more useful imaging technique for judgment of pancreas fat deposition, and a comparison with MRI, which is known to be excellent in judgment of fat deposition, is needed.
Evaluation of pancreas by abdominal sonography is introduced as a screening tool for diagnosis of pancreatic disease by many authors because of its significant accuracy, cost-effectiveness, and few side effects[22]. Therefore, evaluating pancreas echogenicity during abdominal sonography, which is frequently used in health examinations, is expected to play a part as another indicator for screening for metabolic syndrome and insulin resistance.
This study does suffer from a few limitations. Firstly, the fatty pancreas group (n = 180) was larger than the non-fatty pancreas group (n = 113), because it was a retrospective study of limited subjects of a single medical center. Secondly, the hyperinsulinemic euglycemic clamp technique, which is know to be the most accurate evaluation method of insulin resistance, was not used and this needs to be incorporated into in future studies. Thirdly, the degree of fatty pancreas was not well defined, and there might be problems with inter-observer agreement among ultrasonographers when grading different levels of pancreas echogenicity.
An interesting finding of this study is that in a majority of cases (67.9%), fatty pancreas and fatty liver were found concurrently on sonography, and most fatty liver patients (96.9%) also showed fatty pancreas. Although the positive predictive value of fatty liver in fatty pancreas was 69.4%, the negative predictive value of fatty liver in normal pancreas was 96.4%. This implies that fatty pancreas could be used as the initial indicator of ‘ectopic fat deposition’ and as an early marker of insulin resistance, which is a key element of fatty liver and/or metabolic syndrome. More studies will be necessary on the role of fatty pancreas as an early marker of ectopic fat deposition or insulin resistance.
This is the first study that evaluated pancreatic steatosis using sonography and the clinical factors related to it, including its relations with insulin resistance and metabolic syndrome. Additional studies are needed to investigate the actual progress of fatty pancreas into metabolic diseases.
Pathophysiology, diagnostic criteria, and clinical implications of steatosis in liver or fatty liver are already well known. However, there are few studies on the lipid deposition in pancreas (fatty pancreas) and its clinical implications. The authors evaluated the clinical implications of fatty pancreas.
In particular, steatosis in liver or fatty liver refers to fat deposition in hepatocyte, and its pathophysiology, diagnostic criteria, clinical implications are already well known. However, researches on lipid deposition in pancreas (fatty pancreas) and its clinical implications are still insufficient.
Insulin resistance, visceral fat, triglyceride, and alanine aminotransferase (ALT) tended to increase with the degree of fat deposition in the pancreas on sonography. In a multivariate logistic regression analysis, insulin resistance, visceral fat, and ALT level were independently related to fatty pancreas after adjustment for age, body mass index, and lipid profile. Incidence of metabolic syndrome in the fatty pancreas group was significantly higher than in the control group.
A majority of cases (67.9%) of fatty pancreas and fatty liver were found concurrently on sonography. Although positive predictive value of fatty liver in fatty pancreas was 69.4%, the negative predictive value of fatty liver in normal pancreas was 96.4%. This implies the possibility of fatty pancreas as the initial indicator of ‘ectopic fat deposition’ and as an early marker of insulin resistance, which is a key element of fatty liver and/or metabolic syndrome.
The increase in echogenicity of pancreatic body over the kidney echogenicity was classified as fatty pancreas. Fatty pancreas was subdivided into four stages: control group (non-fatty pancreas), where pancreas echogenicity was similar to kidney parenchymal echogenicity; light fatty pancreas, where the pancreas echogenicity was higher than kidney echogenicity, but very much lower than the retroperitoneal fat echogenicity; severely fatty pancreas, where the pancreas echogenicity was higher than kidney echogenicity, but a little lower than the retroperitoneal fat echogenicity; and highly fatty pancreas, where the pancreas echogenicity was similar to the retroperitoneal fat echogenicity.
This study contains useful information on a difficult topic to study; i.e. fatty pancreas. The authors also speculate on a possible relationship between metabolic syndrome and fatty pancreas and/or fatty liver.
| 1. | Yin W, Liao D, Kusunoki M, Xi S, Tsutsumi K, Wang Z, Lian X, Koike T, Fan J, Yang Y. NO-1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet-induced diabetic swine. J Endocrinol. 2004;180:399-408. |
| 2. | Gullo L. Benign pancreatic hyperenzymemia or Gullo's syndrome. JOP. 2006;7:241-242; author reply 243-244. |
| 3. | Nghiem DD, Olson PR, Ormond D. The "fatty pancreas allograft": anatomopathologic findings and clinical experience. Transplant Proc. 2004;36:1045-1047. |
| 4. | Tham RT, Heyerman HG, Falke TH, Zwinderman AH, Bloem JL, Bakker W, Lamers CB. Cystic fibrosis: MR imaging of the pancreas. Radiology. 1991;179:183-186. |
| 5. | Ferrozzi F, Bova D, Campodonico F, De Chiara F, Uccelli M, Bacchini E, Grinzcich R, dè Angelis GL, Battistini A. Cystic fibrosis: MR assessment of pancreatic damage. Radiology. 1996;198:875-879. |
| 6. | Marks WM, Filly RA, Callen PW. Ultrasonic evaluation of normal pancreatic echogenicity and its relationship to fat deposition. Radiology. 1980;137:475-479. |
| 7. | Glaser J, Stienecker K. Pancreas and aging: a study using ultrasonography. Gerontology. 2000;46:93-96. |
| 8. | Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878-10882. |
| 9. | Hirose H, Lee YH, Inman LR, Nagasawa Y, Johnson JH, Unger RH. Defective fatty acid-mediated beta-cell compensation in Zucker diabetic fatty rats. Pathogenic implications for obesity-dependent diabetes. J Biol Chem. 1996;271:5633-5637. |
| 10. | Milburn JL Jr, Hirose H, Lee YH, Nagasawa Y, Ogawa A, Ohneda M, BeltrandelRio H, Newgard CB, Johnson JH, Unger RH. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem. 1995;270:1295-1299. |
| 11. | Gulcan E, Gulcan A, Ozbek O. Is there a role of pancreatic steatosis together with hypertrigliceridemia on the pathogenesis of diabetes in a patient with type 2 diabetes mellitus? Med Hypotheses. 2007;68:912-913. |
| 12. | Gullo L, Salizzoni E, Serra C, Calculli L, Bastagli L, Migliori M. Can pancreatic steatosis explain the finding of pancreatic hyperenzymemia in subjects with dyslipidemia? Pancreas. 2006;33:351-353. |
| 13. | Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495. |
| 14. | Worthen NJ, Beabeau D. Normal pancreatic echogenicity: relation to age and body fat. AJR Am J Roentgenol. 1982;139:1095-1098. |
| 15. | Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727-729. |
| 16. | Quinn SF, Gosink BB. Characteristic sonographic signs of hepatic fatty infiltration. AJR Am J Roentgenol. 1985;145:753-755. |
| 17. | Chalasani N, Deeg MA, Persohn S, Crabb DW. Metabolic and anthropometric evaluation of insulin resistance in nondiabetic patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:1849-1855. |
| 18. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. |
| 19. | Noronha M, Salgadinho A, Ferreira De Almeida MJ, Dreiling DA, Bordalo O. Alcohol and the pancreas. I. Clinical associations and histopathology of minimal pancreatic inflammation. Am J Gastroenterol. 1981;76:114-119. |
| 20. | Isserow JA, Siegelman ES, Mammone J. Focal fatty infiltration of the pancreas: MR characterization with chemical shift imaging. AJR Am J Roentgenol. 1999;173:1263-1265. |
| 21. | Heuck A, Maubach PA, Reiser M, Feuerbach S, Allgayer B, Lukas P, Kahn T. Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18-22. |
| 22. | Alzaid A, Aideyan O, Nawaz S. The size of the pancreas in diabetes mellitus. Diabet Med. 1993;10:759-763. |