Published online Apr 14, 2009. doi: 10.3748/wjg.15.1769
Revised: February 11, 2009
Accepted: February 18, 2009
Published online: April 14, 2009
The occurrence of leiomyoma of the rectum is uncommon. Most of these lesions are clinically silent and are found incidentally during laparotomy or endoscopic procedures for unrelated conditions. Symptomatic leiomyomas of the rectum are encountered less frequently, with only sporadic reports in the literature. We describe a case of a leiomyoma of the rectum presenting as recurrent lower gastrointestinal hemorrhage and secondary anemia.
- Citation: Palma GDD, Rega M, Masone S, Siciliano S, Persico M, Salvatori F, Maione F, Esposito D, Bellino A, Persico G. Lower gastrointestinal bleeding secondary to a rectal leiomyoma. World J Gastroenterol 2009; 15(14): 1769-1770
- URL: https://www.wjgnet.com/1007-9327/full/v15/i14/1769.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1769
Smooth muscle tumors may occur throughout the entire gastrointestinal tract, but are rarely seen in the colon and rectum. Most of these lesions are clinically silent and are found incidentally during laparotomy or endoscopic procedures for unrelated conditions. Symptomatic leiomyomas (LMs) of the rectum are encountered less frequently, with only sporadic reports in the literature. We describe a case of LM of the rectum, presenting as recurrent lower gastrointestinal hemorrhage and secondary anemia.
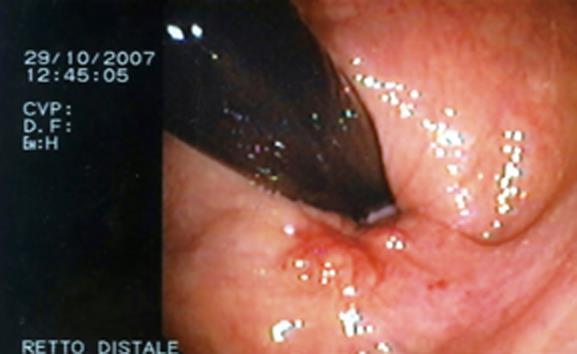
A 55-year-old woman presented to our unit complaining of recurrent rectal bleeding and secondary sideropenic anemia. Colonoscopy revealed the presence of a polypoid, submucous, ulcerated lesion in its vertex (2 cm from the anal margin) (Figure 1).
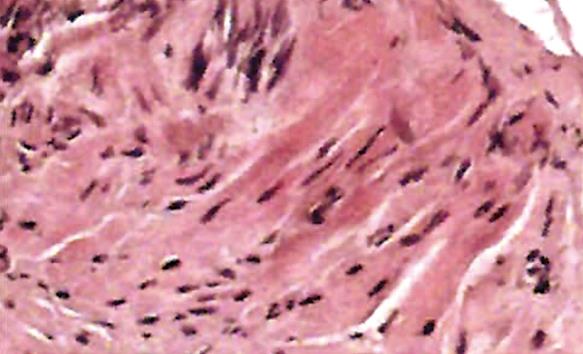
An endoanal ultrasound scan showed a mass located in the anterior wall of the rectum, approximately 7 cm in size, with no infiltration of perirectal fat (Figure 2). A biopsy was made and the pathological study showed a proliferation of fusiform, elongated spindle cells arranged in fascicles. The nuclei were elongated and cigar-shaped, and there was minimal nuclear pleomorphism. No mitotic figures were seen (Figure 3). Immunohistochemistry was positive for smooth muscle actin (SMA) and desmin and negative for CD117.
With a preoperative diagnosis of rectal LM, the mass was removed by local excision with preservation of the rectum. The patient is currently in the 12th mo of follow-up, and has no signs or symptoms of relapse.
Primary LMs present most commonly in the female genital tract and as skin lesions. This tumor is seldom encountered in the gastrointestinal tract. The most common localization is the stomach, followed by the small intestine. The colon, rectum and esophagus are less likely sites. LM of the anorectal region represent 3% of all gastrointestinal LM, and less than 0.1% of rectal tumors[1–6].
Most reported LMs are sessile intraluminal or intramural tumors. They can also present as pedunculated extra luminal mass of the colon[7]. LM often remain asymptomatic until they have reached a fairly large size. The clinical manifestations of these smooth muscle tumors depend on the location, size and direction of tumor growth. They include intestinal obstruction, hemorrhage, and perforation into the peritoneal cavity.
Intraluminal lesions can be detected earlier because of the earlier presentation of symptoms. Many of these tumors are discovered incidentally on routine endoscopic examination of the large bowel. Endoscopically, these tumors can present as pedunculated intramural or intraluminal polyps, and they may look like the more usual adenomas. Complementary investigation, such as with computed tomography, endoscopic ultrasonography, and magnetic resonance imaging, strongly corroborates the diagnosis. Endorectal ultrasound can help to define the extent of disease and may be a useful adjunct in deciding about the appropriate surgical procedure[8].
The biological behavior of smooth muscle tumors varies from benign to locally aggressive and highly malignant. The biological behavior may not be reflected by the histology, as even benign-looking smooth muscle tumors may metastasize. Thus, a combination of site, tumor size, histological appearance and mitotic count give the best prediction of behavior[9].
LM should be separated from gastro-intestinal stromal tumors (GISTs). LMs are positive for actin and desmin and negative for CD34 and CD117 (KIT), and GISTs have the opposite pattern[10]. Surgical excision is the treatment of choice for most LMs. Snare polypectomy is an adequate treatment, but large LMs are believed to be best treated by surgical resection, because conventional colonoscopic resection of large and deep-seeded tumors poses a high risk of perforation[11]. Ensuring the complete removal and follow-up are necessary precautions for tumors with any atypia or mitotic activity.
| 2. | Kusminsky RE, Bailey W. Leiomyomas of the rectum and anal canal: report of six cases and review of the literature. Dis Colon Rectum. 1977;20:580-599. |
| 3. | Stavorovsky M, Morag B, Stavorovsky H, Papo J. Smooth muscle tumors of the alimentary tract. J Surg Oncol. 1983;22:109-114. |
| 4. | He LJ, Wang BS, Chen CC. Smooth muscle tumours of the digestive tract: report of 160 cases. Br J Surg. 1988;75:184-186. |
| 5. | Morgan BK, Compton C, Talbert M, Gallagher WJ, Wood WC. Benign smooth muscle tumors of the gastrointestinal tract. A 24-year experience. Ann Surg. 1990;211:63-66. |
| 6. | Bjornsdottir H, Bjornsson J, Gudjonsson H. Leiomyomatous colonic polyp. Dig Dis Sci. 1993;38:1945-1947. |
| 7. | David SS, Samuel JJ. Pedunculated extraluminal leiomyoma of the sigmoid colon. J Gastroenterol Hepatol. 1996;11:299-300. |
| 8. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. |
| 9. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. |
| 10. | Miettinen M, Sarlomo-Rikala M, Sobin LH. Mesenchymal tumors of muscularis mucosae of colon and rectum are benign leiomyomas that should be separated from gastrointestinal stromal tumors--a clinicopathologic and immunohistochemical study of eighty-eight cases. Mod Pathol. 2001;14:950-956. |
| 11. | Ishiguro A, Uno Y, Ishiguro Y, Munakata A. Endoscopic removal of rectal leiomyoma: case report. Gastrointest Endosc. 1999;50:433-436. |