Published online Apr 14, 2009. doi: 10.3748/wjg.15.1744
Revised: February 7, 2009
Accepted: February 14, 2009
Published online: April 14, 2009
AIM: To investigate the anti-angiogenic and anti-tumor activities of recombinant vascular basement membrane-derived multifunctional peptide (rVBMDMP) in hepatocellular carcinoma (HCC).
METHODS: HepG2, Bel-7402, Hep-3B, HUVE-12 and L-02 cell lines were cultured in vitro and the inhibitory effect of rVBMDMP on proliferation of cells was detected by MTT assay. The in vivo antitumor efficacy of rVBMDMP on HCC was assessed by HepG2 xenografts in nude mice. Distribution of rVBMDMP, mechanism by which the growth of HepG2 xenografts is inhibited, and microvessel area were observed by proliferating cell nuclear antigen (PCNA) and CD31 immunohistochemistry.
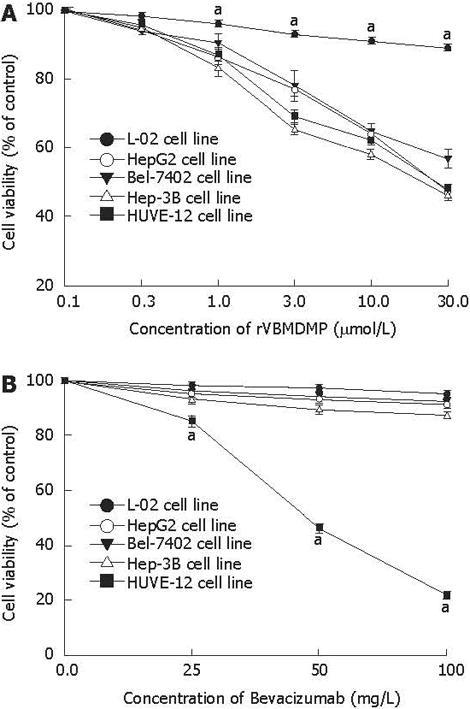
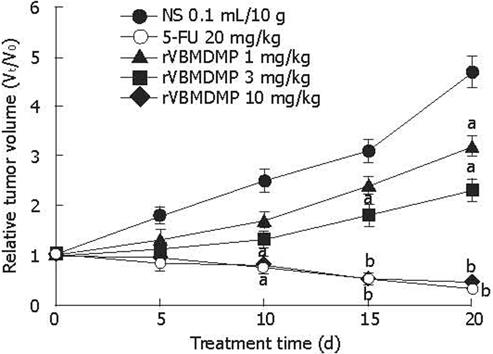
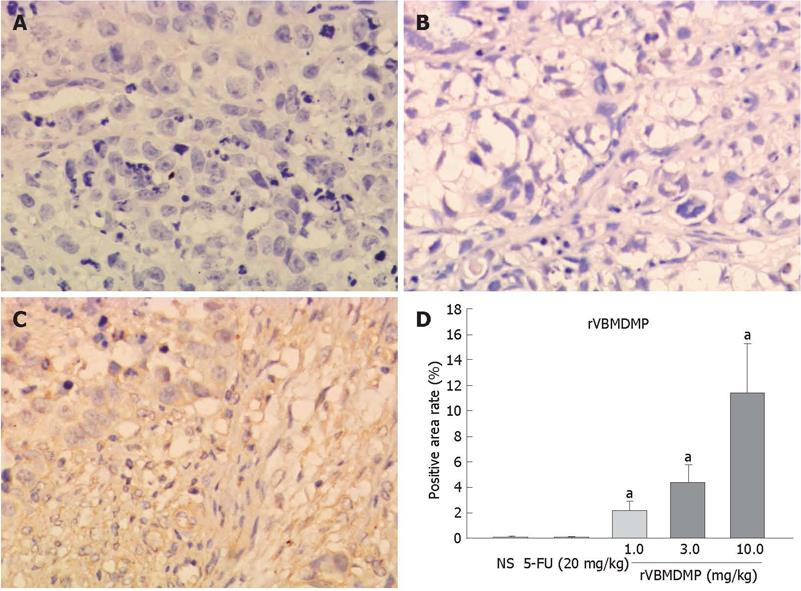
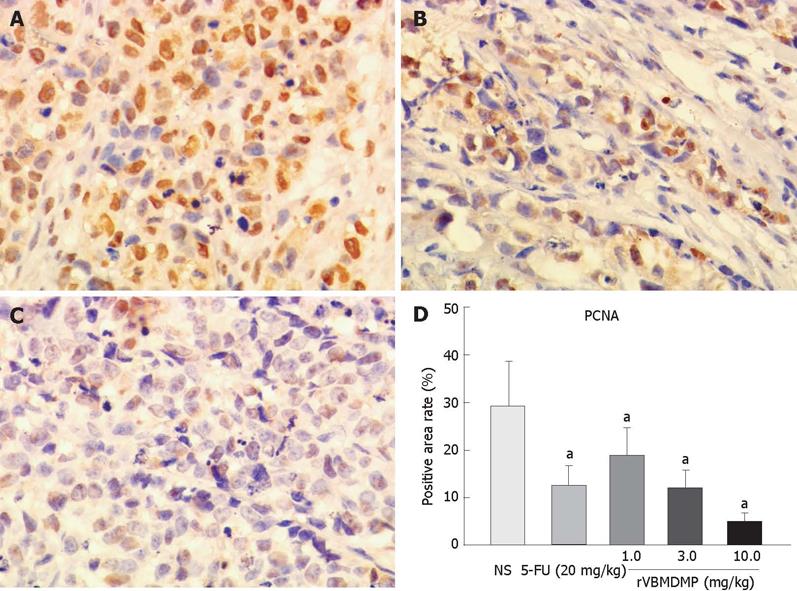
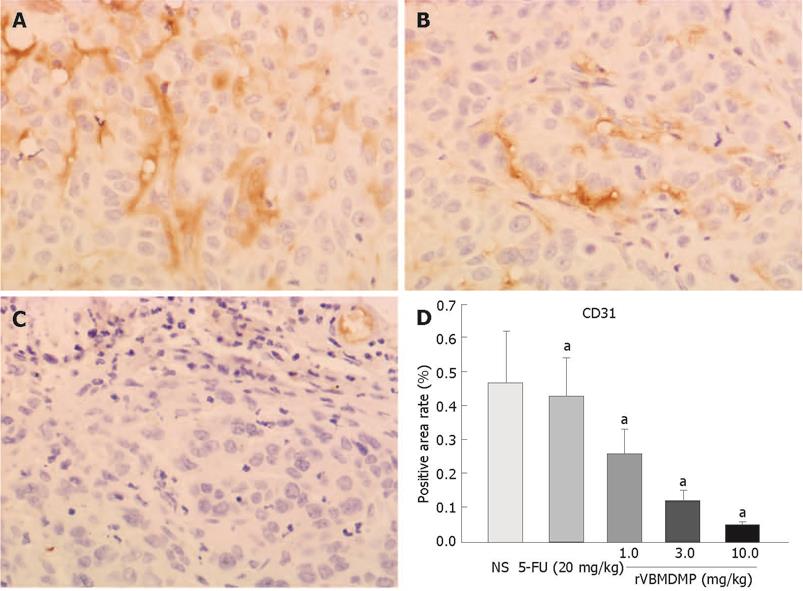
RESULTS: MTT assay showed that rVBMDMP markedly inhibited the proliferation of human HCC (HepG2, Bel-7402, Hep-3B) cells and human umbilical vein endothelial (HUVE-12) cells in a dose-dependent manner, with little effect on the growth of L-02 cells. When the IC50 was 4.68, 7.65, 8.96, 11.65 and 64.82 &mgr;mol/L, respectively, the potency of rVBMDMP to HepG2 cells was similar to 5-fluorouracil (5-FU) with an IC50 of 4.59 &mgr;mol/L. The selective index of cytotoxicity to HepG2 cells of rVBMDMP was 13.8 (64.82/4.68), which was higher than that of 5-FU [SI was 1.9 (8.94/4.59)]. The VEGF-targeted recombinant humanized monoclonal antibody bevacizumab (100 mg/L) did not affect the proliferation of HepG2, Bel-7402, Hep-3B and L-02 cells, but the growth inhibitory rate of bevacizumab (100 mg/L) to HUVE-12 cells was 87.6% ± 8.2%. Alternis diebus intraperitoneal injection of rVBMDMP suppressed the growth of HepG2 xenografts in a dose-dependent manner. rVBMDMP (1, 3, 10 mg/kg) decreased the tumor weight by 12.6%, 55.9% and 79.7%, respectively, compared with the vehicle control. Immunohistochemical staining of rVBMDMP showed that the positive area rates (2.2% ± 0.73%, 4.5% ± 1.3% and 11.5% ± 3.8%) in rVBMDMP treated group (1, 3, 10 mg/kg) were significantly higher than that (0.13% ± 0.04%) in the control group (P < 0.01). The positive area rates (19.0% ± 5.7%, 12.2% ± 3.5% and 5.2% ± 1.6% ) of PCNA in rVBMDMP treated group (1, 3, 10 mg/kg) were significantly lower than that (29.5% ± 9.4%) in the control group (P < 0.05). rVBMDMP at doses of 1, 3 and 10 mg/kg significantly reduced the tumor microvessel area levels (0.26% ± 0.07%, 0.12% ± 0.03% and 0.05% ± 0.01% vs 0.45% ± 0.15%) in HepG2 xenografts (P < 0.01), as assessed by CD31 staining.
CONCLUSION: rVBMDMP has effective and unique anti-tumor properties, and is a promising candidate for the development of anti-tumor drugs.
- Citation: Wu YH, Cao JG, Xiang HL, Xia H, Qin Y, Huang AJ, Xiao D, Xu F. Recombinant vascular basement-membrane-derived multifunctional peptide inhibits angiogenesis and growth of hepatocellular carcinoma. World J Gastroenterol 2009; 15(14): 1744-1750
- URL: https://www.wjgnet.com/1007-9327/full/v15/i14/1744.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1744
Hepatocellular carcinoma (HCC), the fifth most common cancer in the world, is responsible for over 600 000 deaths annually[1]. The majority of patients with HCC die within 1 year after the diagnosis. Unfortunately, HCC is often diagnosed at its late stage when potentially curative therapies are least effective. For such patients, medical treatment modalities, including chemotherapy, chemoembolization, ablation, and proton beam therapy, remain disappointing. Most patients show recurrent HCC that rapidly progresses to its advanced stage with vascular invasion and multiple intrahepatic metastases and their 5-year survival rate is only 7%[2]. Patients with surgically resectable localized HCC have a better prognosis, but their 5-year survival rate is only 15%-39%[3], showing that new therapies for this aggressive disease are urgently needed.
Angiogenesis plays a critical role in the development of HCC. Antiangiogenesis therapy, which inhibits blood vessel formation, may be a promising treatment modality for HCC, because HCC depends on a rich blood supply[4].
Tumstatin, a 28-kDa (244 amino acids) peptide fragment derived from the NC1 domain of α3 chain of type IV collagen, is an endogenous angiogenesis inhibitor, and has two binding sites for avβ3 integrin. One is in the N-terminal region of the molecule consisting of amino acids 74-98, which is associated with the anti-angiogenic property. The other is in the C-terminal region consisting of amino acids 185-203, which is associated with the antitumor activity[5–7]. The peptide fragment of tumstatin consisting of amino acids 74-98 binds to both endothelial and melanoma cells, but only inhibits the proliferation of endothelial cells. However, the anti-tumor activity of amino acids 185-203 is not realized until this peptide region is exposed by truncation, a requirement not essential for the anti-angiogenic activity of amino acids 74-98[5].
By targeting proliferating tumor cells and endothelial cells in a previous study[8], we have constructed a fusion gene of the human IgG3 upper hinge region with two tumstatin-derived specific sequences, which exhibit anti-proliferation and anti-angiogenic activities. The human IgG3 upper hinge region is composed of 11 amino acids, and has a good flexibility, thus not affecting the spatial conformations of the connected peptides. The fusion sequence is named vascular basement membrane-derived multifunctional peptide (VBMDMP)[9]. Recombinant VBMDMP (rVBMDMP) can significantly inhibit tumor growth and metastasis in a mouse lung carcinoma model[10]. Moreover, rVBMDMP selectively inhibits the proliferation of endothelial and human colon cancer cells, as well as induces apoptosis of endothelial cells in vitro and suppresses the growth of human colon cancer xenografts in Balb/c-nude mice[11]. However, whether rVBMDMP inhibits tumor growth and angiogenesis of human HCC xenografts in a nude mouse model is unknown.
In the present study, we showed that rVBMDMP selectively inhibited the proliferation of HCC cells, using in vitro models of tumor growth, and also potently inhibited tumor neoangiogenesis of HepG2 xenografts in a nude mouse model, suggesting that rVBMDMP can be used as a potential agent in the treatment of human HCC.
HepG2, Bel-7402, Hep-3B, HUVE-12 and L-02 cell lines, were purchased from the China Center for Type Culture Collection (CCTCC), were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 &mgr;g/mL streptomycin (Life Technologies) in an incubator containing 50 mL/L CO2 at 37°C. rVBMDMP was over-expressed in Escherichia coli with pGEX-4T-1-VBMDMP and purified as previously described[10] with a purity of over 95%. Synthetic peptide CNYYSNSYSFWLASLNPER (amino acid 185-203 of tumstatin, T4 peptide) and its rabbit polyclonal antibody were provided by Xi’an Huacheng Biotechnology Co., Ltd (China). Bevacizumab was purchased from Roche (Avastin®; Basel, Switzerland). Mouse monoclonal antibodies against proliferating cell nuclear antigen (PCNA) and CD31, as well as peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA).
Cells were seeded in a 96-well plate at a density of 1000 cells/well as described previously[12]. Different concentrations of drugs were added to each well and cultured for 48 h, followed by incubation with 0.5 g/L MTT for 4 h. The supernatant was removed after centrifugation. Finally, 100 &mgr;L DMSO was added and the adsorbance at 570 nm wavelength (A570) was measured with an enzyme-labeling instrument (ELX-800 type). Relative cell proliferation inhibition rate (IR) = (1-average A570 of the experimental group/average A570 of the control group) × 100%. The IR was analyzed using the Calcusyn program to determine the IC50.
Balb/c-nude female mice (Vital River Laboratory Animal Technology Co., Ltd), used in in vivo study, were housed in a sterile room at Institute of Cancer Research, University of South China, with free access to food and water.
Tumors were generated by harvesting HepG2 cells from mid-log phase cultures using 0.25% trypsin (Life Technologies). Cells were resuspended in PBS to a final cell count of 2.5 × 107/mL. A cell suspension (0.2 mL) was subcutaeously injected into the back of each mouse. The mice received a total of 10 injections of 1, 3, and 10 mg/kg body weight rVBMDMP (i.p) every other day when their average tumor volume reached 200 mm3.
Tumor dimensions and body weight were recorded every 5 d from the beginning of treatment. Tumor length and width were measured using a Vernier caliper, and tumor volume was calculated as described previously[13]. Upon termination of treatment, the mice were weighed and sacrificed, and their tumors were excised. The mean tumor weight per group was calculated. The ratio of the mean of the treated tumor weight to the mean of vehicle control tumor weight × 100 was subtracted from 100% to give the tumor growth inhibition rate for each group.
Immunohistochemical staining of paraffin tumor tissue sections was done with rabbit polyclonal anti-T4 peptide antibody (Xi’an Huacheng Biotechnology) at a dilution of 1:50 using the DAB system from DAKO (Carpinteria, CA, USA) according to the manufacturer’s instructions.
The tumor tissue sections were viewed at × 100 magnification and images were captured with a digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA), and analyzed under four fields, excluding peripheral connective tissue and necrotic regions. The total tissue area in each section was 2.576 mm2. Areas of rVBMDMP, PCNA or CD31-positive objects were quantified using ImagePro Plus version 3.0 (Media Cybernetics, Silver Spring, MD, USA). Percentage of microvessel area (MVA) in each field was calculated as (area of CD31-positive objects/measured tissue area) × 100. Percentage of rVBMDMP or PCNA-positive staining in each field was calculated as (area of rVBMDMP or PCNA-positive objects/measured tissue area) × 100. Mean values of MVA- or VBMDMP or PCNA-positive area in each group were calculated from six tumor tissue samples.
Experimental data in each group were expressed as mean ± SD. Analysis of variance was performed with SPSS software for Windows 15.0 using one way ANOVA and pairwise comparison with Student’s t test. P < 0.05 was considered statistically significant.
MTT assay showed that rVBMDMP markedly inhibited the proliferation of human HCC (HepG2, Bel-7402, Hep-3B) cells and human umbilical vein endothelial (HUVE-12) cells in a dose-dependent manner, with little effect on the growth of L-02 cells (Figure 1A). When the IC50 was 4.68, 7.65, 8.96, 11.65 and 64.82 &mgr;mol/L, respectively, the potency of rVBMDMP to HepG2 cells was similar to that of 5-FU with an IC50 of 4.59 &mgr;mol/L. The selective index of cytotoxicity to HepG2 cells of rVBMDMP was 13.8 (64.82/4.68), which was higher than that of 5-FU with a SI of 1.9 (8.94/4.59). Bevacizumab (100 mg/L) did not affect the proliferation of HepG2, Bel-7402, Hep-3B and L-02 cells, but its growth inhibitory rate for HUVE-12 cells was 87.6% ± 8.2% (Figure 1B).
rVBMDMP inhibited the growth of implanted HepG2 tumor xenografts in nude mice in a dose-dependent manner (Figure 2). Different doses of rVBMDMP (1, 3, 10 mg/kg) decreased the tumor weight by 12.6%, 55.9% and 79.7%, respectively, compared with the vehicle control.
Immunohistochemical staining of rVBMDMP showed that the positive area rates (2.2% ± 0.73%, 4.5% ± 1.3% and 11.5% ± 3.8%) were significantly higher in rVBMDMP treated group (1, 3, 10 mg/kg) than that (0.13% ± 0.04%) in the control group (P < 0.01, Figure 3), indicating that rVBMDMP can accumulate in HepG2 xenografts nude mice.
After intraperitoneal injection of rVBMDMP every other day, the positive area rates (19.0% ± 5.7%, 12.2% ± 3.5% and 5.2% ± 1.6%) of PCNA in rVBMDMP treated group (1, 3, 10 mg/kg) were significantly lower than that in the control group (29.5% ± 9.4%) (P < 0.05, Figure 4), suggesting that rVBMDMP inhibits the proliferation of tumor cells in HepG2 xenografts in nude mice.
The tumor MVA rates (0.26% ± 0.07%, 0.12% ± 0.03% and 0.05% ± 0.01%) were significantly lower in the HepG2 xenografts of the rVBMDMP-treated group assessed by CD31 staining (1, 3 and 10 mg/kg) than that (0.45% ± 0.15%) in the control group (P < 0.01, Figure 5), demonstrating that rVBMDMP inhibits angiogenesis of HepG2 xenografts in nude mice.
It was recently reported that angiogenesis inhibitors may not work well in monotherapy[1415]. In contrast, studies conducted in preclinical tumor models showed that angiogenesis inhibitors in combination with cytotoxic chemotherapeutic agents or radiation therapy produce additive or synergistic anti-tumor activities[121617]. The positive effects of combined chemotherapy with angiogenesis inhibitors have been reported[18–22], suggesting that the combination therapy of a cytotoxic agent and an angiogenesis inhibitor may be a fruitful topic in future clinical research[2324].
In this report, rVBMDMP inhibited the proliferation of human HCC cells selectively in vitro. Our previously research also showed that rVBMDMP could inhibit the proliferation of colon caner cells, but have no effect on the proliferation of normal cells[11], suggesting that rVBMDMP can maintain the selective anti-tumor activity of tumstatin amino acids 185-203 fragment, which is consistent with the previously reported findings[23]. The specific inhibitory effect of rVBMDMP on the proliferation of tumor cells strongly suggests that rVBMDMP functions via a tumor-specific cell surface protein or its receptor.
Tumor neoangiogenesis has recently been recognized as an important factor in defining subsets of cancer patients with a poor outcome[25–27]. A number of angiogenesis inhibitors, discovered in recent years, can inhibit tumor growth by targetting proliferating and migrating ECs. Targeting ECs supports growth of tumor rather than tumor cells directly, which is particularly promising because these ECs are genetically stable and do not develop drug resistance. In this study, rVBMDMP suppressed reduplication in human endothelial HUVE-12 cells, like bevacizumab. By immunostaining of CD31 in tumor tissues, we found that rVBMDMP significantly decreased the microvessel density of human HCC xenografts in a mouse model. It was reported that rVBMDMP can significantly inhibit the proliferation of endothelial cells, blood vessel formation, and tumor growth in in vitro and in vivo models of angiogenesis, as well as induce EC-specific apoptosis[11]. These anti-angiogenic properties of rVBMDMP, coupled with its anti-tumor activities, strongly indicate that rVBMDMP acts as a novel inhibitor of angiogenesis and tumor growth.
Since the proliferation velocity of ECs is higher in tumor tissue than in normal tissue, angiogenesis inhibitors may be accumulated in tumor[28]. Our results show that rVBMDMP was significantly accumulated in human HCC xenografts in a mouse model, indicating that rVBMDMP is selectively distributed in tumor tissue.
Maeshima et al[5] demonstrated that tumstatin amino acids 185-203 fragment does not show anti-tumor activity until the peptide region is exposed to truncation, which is not required for the anti-angiogenic activity of tumstatin amino acids 74-98 fragment. A shorter fragment comprising seven N-terminal residues 185-191 (CNYYSNS) shares the same inhibitory profile. The three-dimensional structures of CNYYSNS and tumstatin amino acids 185-203 fragment show a β-turn at the YSNS (188-191) sequence level, which is crucial for its biological activity[29]. In our study, analysis of the structures of rVBMDMP using the Antheprot software indicated that both ends of the IgG3 upper hinge region sequence were a rarefaction structure, suggesting that rVBMDMP acts as a potent and specific agent against tumor progression[11].
It has been shown that aVβ3 integrin is a putative receptor of tumstatin[3031]. Tumstatin fails to suppress neovascularization of Matrigel plugs in β integrin-deficient mice, and tumors in β integrin-deficient mice grow much faster than tumors in wild-type mice[3031], strongly suggesting that tumstatin acts via aVβ3 integrin as a negative regulator of angiogenesis. We speculate that the anti-tumor activity of rVBMDMP might also be mediated by aVβ3 integrin[32].
In conclusion, rVBMDMP is a novel inhibitor of angiogenesis and tumor growth. Targeting both endothelial and tumor cells can enhance the efficacy of anti-tumor therapy. The mechanism of its action requires further investigation.
Tumstatin, a 28-kDa (244 amino acids) peptide fragment, derived from the NC1 domain of α3 chain of type IV collagen, is an endogenous angiogenesis inhibitor. Tumstatin has two binding sites for avα3 integrin. One is in the N-terminal region of the molecule consisting of amino acids 74-98, which is associated with the anti-angiogenic property. The other is in the C-terminal region consisting of amino acids 185-203, which is associated with the antitumor activity. However, the anti-tumor activity of amino acids 185-203 is not realized until this peptide region is exposed to truncation, a requirement not essential for the anti-angiogenic activity of amino acids 74-98.
Angiogenesis plays a critical role in the development of hepatocellular carcinoma (HCC). Antiangiogenesis therapy, which inhibits blood vessel formation, may be promising treatment modality for HCC, because HCC depends on a rich blood supply. The strategy of targeting both proliferating tumor and endothelial cells can improve the effectiveness of therapy for HCC.
It was recently reported that rVBMDMP significantly inhibits tumor growth and metastasis in a mouse lung carcinoma model and selectively inhibits the proliferation of endothelial and human colon cancer cells. In this study study, recombinant vascular basement membrane-derived multifunctional peptide (rVBMDMP) selectively inhibited the proliferation of HCC cells in in vitro and in vivo models of tumor growth. Furthermore, our in vivo studies suggested that rVBMDMP was significantly accumulated in human HCC xenografts and potently inhibited tumor neoangiogenesis in HepG2 xenografts in nude mice.
rVBMDMP is a novel inhibitor of angiogenesis and tumor growth. Targeting both endothelial and tumor cells can enhance the efficacy of anti-tumor therapy and can be used as a treatment modality for HCC.
VBMDMP is a fusion gene in the human IgG3 upper hinge region with two tumstatin-derived specific sequences (amino acids 74-98 and amino acids 185-203), which exhibits anti-proliferation and anti-angiogenic activities. Recombinant VBMDMP (rVBMDMP) is produced as a recombinant molecule in E.coli.
The authors demonstrated that rVBMDMP selectively inhibited the proliferation of HCC cells, and was significantly accumulated in human HCC xenografts, and potently inhibited tumor neoangiogenesis in HepG2 xenografts in a nude mouse model by examining the effects of rVBMDMP on tumor growth and angiogenesis of HCC in vitro and in vivo. The results are interesting and may represent the strategy of targeting both proliferating tumor and endothelial cells, and provide a new treatment modality for HCC.
| 2. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. |
| 3. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. |
| 5. | Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340-21348. |
| 6. | Shahan TA, Ziaie Z, Pasco S, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Identification of CD47/integrin-associated protein and alpha(v)beta3 as two receptors for the alpha3(IV) chain of type IV collagen on tumor cells. Cancer Res. 1999;59:4584-4590. |
| 7. | Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745-23750. |
| 8. | Pack P, Müller K, Zahn R, Plückthun A. Tetravalent miniantibodies with high avidity assembling in Escherichia coli. J Mol Biol. 1995;246:28-34. |
| 9. | Peng SP, Fang WY, Dai WJ, Zou XQ, Liu HY, Shi SH, Cao JG. Cloning, expression and space conformation analysis of vascular basement membrane-derived multifunctional peptide. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 2003;10:185-189. |
| 10. | Peng SP, Fang WY, Jiang RC, Zhou JG, Dong L, Cao JG. Prokaryotic expression of vascular basement membrane-derived multifunctional peptide and its anti-tumor activity assay. Zhongguo Yaolixue Tongbao. 2003;19:678-682. |
| 11. | Cao JG, Peng SP, Sun L, Li H, Wang L, Deng HW. Vascular basement membrane-derived multifunctional peptide, a novel inhibitor of angiogenesis and tumor growth. Acta Biochim Biophys Sin (Shanghai). 2006;38:514-521. |
| 12. | Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287-291. |
| 13. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. |
| 14. | Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526-1528. |
| 15. | Liu W, Ahmad SA, Reinmuth N, Shaheen RM, Jung YD, Fan F, Ellis LM. Endothelial cell survival and apoptosis in the tumor vasculature. Apoptosis. 2000;5:323-328. |
| 16. | Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW Jr. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369-3372. |
| 17. | Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190-2196. |
| 18. | Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996-2003. |
| 19. | Reimer CL, Agata N, Tammam JG, Bamberg M, Dickerson WM, Kamphaus GD, Rook SL, Milhollen M, Fram R, Kalluri R. Antineoplastic effects of chemotherapeutic agents are potentiated by NM-3, an inhibitor of angiogenesis. Cancer Res. 2002;62:789-795. |
| 20. | Li D, Williams JI, Pietras RJ. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene. 2002;21:2805-2814. |
| 21. | Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15-R24. |
| 22. | Siemann DW, Mercer E, Lepler S, Rojiani AM. Vascular targeting agents enhance chemotherapeutic agent activities in solid tumor therapy. Int J Cancer. 2002;99:1-6. |
| 23. | Semba T, Funahashi Y, Ono N, Yamamoto Y, Sugi NH, Asada M, Yoshimatsu K, Wakabayashi T. An angiogenesis inhibitor E7820 shows broad-spectrum tumor growth inhibition in a xenograft model: possible value of integrin alpha2 on platelets as a biological marker. Clin Cancer Res. 2004;10:1430-1438. |
| 24. | Gasparini G, Longo R, Fanelli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol. 2005;23:1295-1311. |
| 25. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. |
| 26. | Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964-3968. |
| 27. | Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol. 1998;184:119-122. |
| 28. | Huynh H, Chow PK, Palanisamy N, Salto-Tellez M, Goh BC, Lee CK, Somani A, Lee HS, Kalpana R, Yu K. Bevacizumab and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Hepatol. 2008;49:52-60. |
| 29. | Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem. 2001;276:15240-15248. |
| 30. | Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589-601. |