INTRODUCTION
Reactive oxygen species (ROS) are molecules or ions formed by the incomplete one-electron reduction of oxygen. These reactive oxygen intermediates include singlet oxygen, superoxides, peroxides, hydroxyl radical, and hypochlorous acid. They contribute to the microbicidal activity of phagocytes, regulation of signal transduction and gene expression, and induce oxidative damage to nucleic acids, proteins, and lipids. Peroxidation by ROS alters the amounts of unsaturated fatty acids and proteins in the cell membrane and thus affects membrane fluidity. In addition, with aging, humans tend to show an increased affectability of lipid peroxides caused by ROS[1]. Recent research has indicated that ROS also play a critical role in the energy dysfunction of mitochondria caused by ethanol-induced gastric mucosa injury[2]. In addition, oxidative damage caused by ROS and other free radicals is involved in a number of pathological conditions including cancer. Data presented herein is consistent with this opinion. Yagoda et al[3] found erastin interacted with voltage-dependent anion channel proteins to induce mitochondrial dysfunction, release of oxidative species and, ultimately, non-apoptotic, oxidative cell death. This process has a degree of selectivity for cells with activated Ras-Raf-MEK signaling. ROS production also involves the induction of autophagy, which contributes to caspase-independent macrophage cell death[4]. ROS, produced in the redox cycle, contribute to p53 mutations, which are dominated by G-to-T transversions. These mutations are suppressed by ROS attenuators[56]. The mutations of p53, a well characterized tumor suppressor, are believed to relate to carcinogenesis. Since oxidative stress comprehensively damages cells and tissues, it is reasonable that factors which induce ROS would contribute to the occurrence and development of tumors, while antioxidant agents that scavenge ROS may inhibit this process. To the best of our knowledge, the former inference is consistent with previous reports[78], however, there is little supportive evidence for the latter[910]. Vitamins C and E, two reducing agents and antioxidants, show no additional benefit in the chemoprevention of gastric cancer[1112]. Inadequate dose, heterogeneous research, poor compliance and multiple effects of antioxidants may lead to this paradox. Is there anything more to be elucidated on this subject?
REGULATION OF ROS PRODUCTION BY RAS
Oxidative stress and Ras activation lead to the production of ROS[13]. Introduction of ROS by Ras may occur at the transcription level. GATA-6 is a component of the specific protein-DNA complexes at the nicotinamide adenine dinucleotide phosphate oxidase (Nox) 1 promoter, and is able to trans-activate the Nox1 promoter. GATA-6 is phosphorylated at serine residues by MEK-activated extracellular signal-regulated kinase (ERK), which enhances GATA-6 DNA binding. The site-directed mutation of the consensus ERK phosphorylation site (PYS(120)P to PYA(120)P) of GATA-6 abolishes its trans-activation activity, suppressing the growth of CaCo-2 cells. By MEK-ERK-dependent phosphorylation of GATA-6, oncogenic Ras signaling enhances the transcription of Nox1[14]. A regulatory subunit, Rac, of the NADPH oxidase complex also involves the regulation of ROS[15]. Other factors that regulate the production of ROS will not be discussed here.
ROS INVOLVE TUMORIGENESIS THAT RELATES TO THE RAS-RAF-MEK-ERK PATHWAY
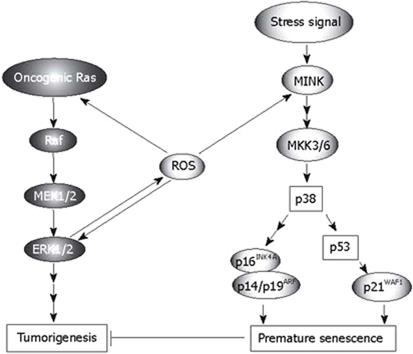
Growth factors, cellular stress, and γ radiation stimulate oncogenic Ras-Raf-MEK signaling, which plays a crucial role in tumorigenesis. As an important mediator of physiological and pathological signal-transduction pathways, ROS is also involved in Ras-Raf-MEK signaling (Figure 1). The functions of ROS in tumorigenesis relating to this pathway include the following. (1) In cells with activated Ras-Raf-MEK signaling, released ROS cause non-apoptotic, oxidative cell death, as previously mentioned[3], and the Ras-ERK pathway is critical in mediating protection against apoptotic cell death induced by increased oxidative stress[16]. (2) The activity of the ROS-generating enzyme Nox1 is required for vascular endothelial growth factor (VEGF), a potent stimulator of tumor angiogenesis. However, if extracellular signal-regulated kinase (ERK)-dependent phosphorylation of the transcription factor Sp1 and Sp1 binding to a VEGF promoter is inhibited, this activity does not occur. Nox1 mediates oncogenic Ras-induced upregulation of VEGF and angiogenesis by activating Sp1 through Ras-ERK-dependent phosphorylation of Sp1[8]. (3) Ras (p19) interaction with p73β, a structural and functional homolog of p53, amplifies p73β-induced apoptotic signaling responses including Bax mitochondrial translocation, cytochrome c release, increased production of ROS and loss of mitochondrial transmembrane potential. After taxol treatment, endogenous expression of Ras and p73β significantly increase, and taxol-enhanced endogenous p73β transcriptional activities are further amplified by p19, which markedly increases cellular apoptosis in the p53-null SAOS2 cancer cell line[17]. (4) In human U937 monocytes, hydrogen peroxide (H2O2) evokes Ca2+ influx through TRPM2 to activate Ca2+-dependent tyrosine kinase Pyk2 and amplify ERK signaling via Ras GTPase. TRPM2 Ca2+ influx controls the ROS-induced signaling cascade responsible for chemokine production, which aggravates inflammation[18].
Figure 1 Roles of ROS in the Ras-Raf-MEK-ERK signaling and p38 pathway (Modified from Ref.
31, with permission).
In contrast, the released ROS have complicated effects on Ras-Raf-MEK signaling, which may occur on several levels (Figure 1). ROS directly enhance the activation of Ras[19], and augment ERK1/2[20]. Melatonin, a natural antioxidant, inhibits the activation of Ras in H4IIE hepatoma cells[21]. Generation of ROS is required for Ras transformation phenotypes including anchorage-independent growth, morphological transformation, and tumorigenesis[22]. In diabetes-related angiogenesis of the retina, activation of H-Ras and its downstream signaling pathway may be under the control of superoxide, and H-Ras activation in diabetes can be prevented by inhibiting superoxide accumulation[23]. H2O2 activates H-Ras and its downstream signaling pathway, including Raf-1 and phosphorylation of p38 MAP kinase. Inhibition of superoxide significantly attenuates glucose-induced activation of H-Ras, Raf-1 and p38 MAP kinase[23]. PI3K is a mediator in the E-Ras-PI3K-Akt signaling pathway, which leads to tumor-like properties in embryonic stem cells. AKR1C2 and AKR1C3 mediated prostaglandin D(2) metabolism augments the PI3K/Akt proliferative signaling pathway in human prostate cancer cells[24]. Activated Ras is usually associated with cancer, but it also produces paradoxical premature senescence in primary cells by inducing ROS followed by the accumulation of tumor suppressors p53 and p16INK4A[25].
ROS INVOLVE TUMOR SUPPRESSION VIA THE P38 PATHWAY
Oncogenic Ras sequentially activates MEK, p38 and two p38 downstream kinases, MAPK-activated protein kinase2 (MK2) and p38 regulated/activated protein kinase (PRAK), which in turn suppress Ras-induced cell proliferation by blocking activation of Jun N-terminal kinase (JNK). Increased intracellular levels of ROS, induced by the Ras-Raf-MEK-ERK signaling cascade, may mediate the activation of the p38 pathway and act as an intermediate signal between the MEK-ERK and MKK3/6-p38 pathways (Figure 1). On the one hand, the activation of p38 mitogen-activated protein kinase (MAPK) is a prerequisite for ROS-mediated functions such as apoptotic cell death in cancer cells[26], and adrenal steroidogenesis[27]. On the other hand, inhibiting or scavenging ROS may attenuate the activation of p38-dependent pathways[2829]. Since Ras induces the production of ROS and the latter activates p38, a conclusion can be derived theoretically that the inhibition of Ras may weaken the tension of p38. This inference is supported by research which involved H4IIE hepatoma cells[21]. However, in some cases, it is not certain that increased intracellular ROS should enhance the activation of p38[30]. The p38 MAPK pathway negatively regulates cell proliferation and tumorigenesis. The involvement of the p38 pathway in the regulation of cellular processes that directly contribute to tumor suppression includes oncogene-induced senescence (OIS), replicative senescence, contact inhibition and DNA-damage responses, which have been discussed in detail[31]. Recently, we found that p38 also plays an important role in inflammation-induced cellular senescence[32], which is believed to be a process related to tumor suppression. Several reports have shown that ROS mediate OIS via p38-dependent pathways[33–35]. The accumulation of intracellular ROS induced by oncogenic Ras is ERK-dependent during the activation of p38 and the induction of senescence. After sensing the oxidative stress induced by activated Ras, p38 directs cells to undergo apoptosis[36]. Human cancer cell lines with high ROS levels display enhanced tumorigenicity and impaired p38α activation by ROS. p38α has also been reported to antagonize oncogenic transformation induced by activated N-Ras in murine fibroblasts[37] and by activated K-Ras in colon cancer cell lines[38]. Activated components of the p38 pathway phosphorylate multiple residues on p53, including Ser33 and Ser46 (by p38), Ser37 (by PRAK), and possibly others, leading to increased transcriptional activity of p53 and induction of a transcriptional target of p53 and p21WAF1[31]. Through an unknown mechanism, activated p38 also induces the expression of p16INK4A and p14/p19ARF, which, together with the p53-p21WAF1 cascade, cause premature senescence that serves as a tumor-suppressing defense mechanism both in cell culture and in vivo[31]. Ras also involves senescence, in which Seladin-1 acts as a key mediator of oxidative stress[39]. Seladin-1 has previously been implicated in Alzheimer’s disease and cholesterol metabolism. Following oncogenic and oxidative stress, Seladin-1 binds to the p53 amino terminus and displaces E3 ubiquitin ligase Mdm2 from p53, thus resulting in p53 accumulation. Ablation of Seladin-1 causes the bypass of Ras-induced senescence in rodent and human fibroblasts, and allows Ras to transform these cells. Wild-type Seladin-1, but not mutants that disrupt its association with either p53 or Mdm2, suppresses the transformed phenotype. The same mutants are also inactive in directing the p53-dependent oxidative stress response[39]. p38 related replicative senescence, contact inhibition and DNA-damage responses will not be discussed here, refer to Ref. 31.
ROS INVOLVE APOPTOSIS THAT RELATES TO THE P38 PATHWAY
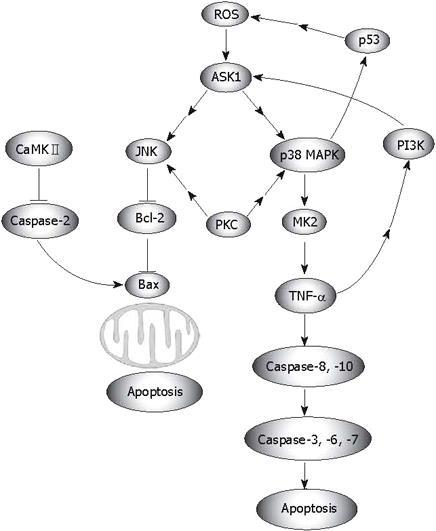
Numerous researchers have shown that ROS relate to apoptosis that is processed through the mitochondrial pathway, which depends on the activation of p38 (Figure 2)[40–44]. Apoptosis signal-regulating kinase 1 (ASK1) is an evolutionarily conserved mitogen-activated protein 3-kinase that activates both JNK and p38 MAPK, which may also be triggered by ROS[45–49]. However, activation of MAPKs (JNK, p38, ERK) is differentially regulated by cleavage size (40 kDa and 36 kDa) of mammalian sterile 20-like kinase 1, which is controlled by caspase-7 and -3[50]. ASK1-induced and ROS-dependent activation of MAPKs is crucial for apoptosis[4351], and for TLR4-mediated mammalian innate immunity[52]. In the case of oxidative stress, a positive feedback may form in the ASK1-p38-TNF-α pathway, which enhances ROS-mediated apoptosis (Figure 2). Ask1 activates both JNK and p38 MAPK, then the activated p38 translocates into the nucleus and stimulates the expression of MK2. After moving out of the nucleus, MK2 increases TNF-α production. On the other hand, enhanced TNF-α and ROS activate ASK1 activity[46], which leads to the activation of JNK. JNK abrogates Bcl-2, which is believed to be a protector away from mitochondria-related apoptosis, although Bcl-2 may manifest opposing phenotypes in text of interacting with other proteins[53]. In addition, this positive feedback is required for ROS-mediated apoptosis (Figure 2). Functional analyses have revealed that the initial ROS-independent activations of JNK, Bax, and caspase-3 are not sufficient for cell death, and thus, should be re-activated by ROS in order to kill the cells[54]. ROS do not simply mediate the lethal action of γ radiation, but actually amplify it by forming a feedback loop between a downstream effector (caspase) and the upstream initiation signals leading to the activation of JNK. This role of ROS appears to allow Bcl-2 to block the signaling events, which are initially induced upstream[54]. p38α MAPK contributes to the further activation of p53, which also leads to a positive feedback loop, p38α MAPK/p53. The p53/ROS/p38α MAPK cascade is essential for cisplatin-induced cell death in HCT116 cells, and the subsequent p38α/p53 positive feedback loop strongly enhances the initial p53 activation[55]. Of interest, p38 may stimulate indirectly the production of ROS via p53. Glioma pathogenesis-related protein 1 (GLIPR1), a novel p53 target gene, is down-regulated by methylation in prostate cancer and has p53-dependent and -independent pro-apoptotic activities in tumor cells. Overexpression of GLIPR1 in cancer cells leads to suppression of colony growth and induction of apoptosis. Mechanistic analysis indicates that GLIPR1 up-regulation increases the production of ROS, leading to apoptosis through activation of the JNK signaling cascade[56]. However, in p38-related apoptosis that is independent of ROS generation, JNK seems to execute a reverse function. Inhibition of JNK by SP600125 significantly enhanced apoptosis[57].
Figure 2 ROS-mediated apoptosis.
Via regulation of MAPKs, the protein kinase C (PKC)δ-mediated pathway also involves ROS related apoptosis. As a tentative stimulator of p38, JNK1/2 and MEK/ERK signaling[58], PKC-δ regulates cell apoptosis and survival in diverse cellular systems. Knock down of PKC-δ suppresses p38 MAPK phosphorylation. Via p38 MAPK, activated PKC-δ regulates the phosphorylation of heat shock protein (HSP) 27. Attenuated phosphorylation of HSP27 correlates with tumor progression in patients with hepatic cell cancer[59]. PKC-δ translocates to different subcellular sites in response to apoptotic stimuli. The localization of PKC-δ differentially affects the activation of downstream signaling pathways. PKC-δ-cytosol increases the phosphorylation of p38, whereas PKC-δ-nucleus increases c-JNK phosphorylation. Moreover, p38 phosphorylation plays a role in the apoptotic effect of PKC-δ-cyto, whereas c-JNK activation mediates the apoptotic effect of PKC-δ-Nuc[60]. Recent evidence has shown that calcium/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII) activity is also enhanced by pro-oxidant conditions. CaMKII is activated by angiotensin II-induced oxidation, leading to apoptosis in cardiomyocytes both in vitro and in vivo (Figure 2)[61].
Besides apoptosis, ROS also relate to proliferation. In mice lacking Nrf2 transcription factor, oxidative stress-mediated activation of p38, Akt kinase and downstream targets is impaired, resulting in enhanced death and delayed proliferation of hepatocytes[62]. p38 MAPK, p53, and p21 also act as molecular mediators on the way from increased ROS levels to the observed growth arrest[63].
CONCLUSION
Besides their well-known roles, recent studies have demonstrated additional functions of ROS in tumorigenesis. However, the evidence comes from studies performed in cell culture, in addition to data from human tumors. In addition, these cell lines are generally kept in room air, whereas hyperoxic oxygen levels may favor enhanced ROS formation, which is well known. The relevance of ROS in all these events in vivo, especially in humans, is not clear. ROS seem to have dual roles in tumorigenesis, cancer promoting and cancer suppressing. ROS participate in both Ras-Raf-MEK1/2-ERK1/2 signaling and the p38 MAPK pathway. However, these two pathways may have inverse functions in tumorigenesis. The former is related to cancer promotion, whereas the latter is associated with a variety of cellular responses such as OIS, replicative senescence, contact inhibition and DNA-damage responses. Thus, regarding ROS as an absolute “carcinogenic factor” or “cancer suppressor” seems to be inappropriate. It seems that more extensive investigations are needed to determine the integrity of ROS in human cancer development. Two aspects of research remain to be carried out in the future. Firstly, we should determine whether ROS directly mediate the Ras-Raf-MEK1/2-ERK1/2 and p38 MAPK signaling pathways, or whether other mediators are needed. Secondly, the definite shunting mechanism, which controls the steering from triggering Ras-Raf-MEK1/2-ERK1/2 signaling to triggering p38 MAPK signaling and vice versa, should be determined. The relationship between ROS and p38-pathway-mediated OIS is of particular interest because several reports indicate that part of the OIS pathway is intact at least in certain cancer cells, and that senescence responses improve the outcome of chemotherapy. Drugs which artificially trigger senescence in tumor cells will thus improve cancer treatment[31]. Studies on the shunting mechanism would facilitate research on the roles of ROS in tumorigenesis, and could shed light on drug discovery.
Supported by National Natural Science Foundation of China, No. 30750013 and Key Science Research Project Natural Science Foundation of Xiamen, No. WKZ0501