Published online Apr 7, 2009. doi: 10.3748/wjg.15.1630
Revised: March 9, 2009
Accepted: March 16, 2009
Published online: April 7, 2009
AIM: To explore the feasibility of passage of bone-marrow-derived liver stem cells (BDLSCs) in culture systems that contain cholestatic serum.
METHODS: Whole bone marrow cells of rats were purified with conditioning selection media that contained 50 mL/L cholestatic serum. The selected BDLSCs were grown in a proliferating culture system and a differentiating culture system. The culture systems contained factors that stimulated the proliferation and differentiation of BDLSCs. Each passage of the proliferated stem cells was subjected to flow cytometry to detect stem cell markers. The morphology and phenotypic markers of BDLSCs were characterized using immunohistochemistry, reverse transcription polymerase chain reaction (RT-PCR) and electron microscopy. The metabolic functions of differentiated cells were also determined by glycogen staining and urea assay.
RESULTS: The conditioning selection medium isolated BDLSCs directly from cultured bone marrow cells. The selected BDLSCs could be proliferated for six passages and maintained stable markers in our proliferating system. When the culture system was changed to a differentiating system, hepatocyte-like colony-forming units (H-CFUs) were formed. H-CFUs expressed markers of embryonic hepatocytes (alpha-fetoprotein, albumin and cytokeratin 8/18), biliary cells (cytokeratin 19), hepatocyte functional proteins (transthyretin and cytochrome P450-2b1), and hepatocyte nuclear factors 1α and -3β). They also had glycogen storage and urea synthesis functions, two of the critical features of hepatocytes.
CONCLUSION: BDLSCs can be selected directly from bone marrow cells, and pure BDLSCs can be proliferated for six passages. The differentiated cells have hepatocyte-like phenotypes and functions. BDLSCs represent a new method to provide a readily available alternate source of cells for clinical hepatocyte therapy.
- Citation: Cai YF, Chen JS, Su SY, Zhen ZJ, Chen HW. Passage of bone-marrow-derived liver stem cells in a proliferating culture system. World J Gastroenterol 2009; 15(13): 1630-1635
- URL: https://www.wjgnet.com/1007-9327/full/v15/i13/1630.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1630
About a decade ago, many reports highlighted the broad developmental potential of bone-marrow-derived stem cells[1]. This gave new hope for cell therapy using autologous bone marrow cells, which has few ethical problems and has been applied to severe liver diseases[2]. However, little progress has been made in recent years because of the difficulties of selection and proliferation of this specific cell population[3]. It is necessary to find a new way to isolate and purify bone-marrow-derived liver stem cells (BDLSCs). Following the principle that cells in culture can survive only when they become accommodated to the surrounding environment, we developed a culture system to isolate BDLSCs from bone marrow cells. Within this system, only BDLSCs could survive, while the other bone marrow cells could not[4]. This method could provide pure BDLSCs, but still could not harvest large numbers of cells, and the efficiency of differentiation was insufficient for therapeutic application. Here, we developed a proliferating culture system to passage BDLSCs and a differentiating system to yield hepatocyte-like cells, by modified culture environments.
Selection, proliferation and differentiation of BDLSCs
Preparation of conditioning selection medium: Common bile duct ligation and transection were carried out under general (ether) anesthesia in Sprague-Dawley rats weighing 200-250 g to induce cholestasis. After 10 d, whole blood was collected from each rat, and serum was separated. Cholestatic serum (50 mL/L) was added to Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) that contained 20 mmol/L HEPES (Sigma), 10-7 mol/L dexamethasone (Sigma), and antibiotics, to act as the conditioning selection medium (See our previous study)[4].
Culture of bone marrow cells: Rat bone marrow cells were obtained by flushing the femurs. About 3 × 108 bone marrow cells were retrieved from one rat. Bone marrow cells were suspended in DMEM and plated at a density of 1 × 109 cells/L onto culture dishes. DMEM enriched with 100 mL/L fetal bovine serum (FBS; Hyclone), 20 mmol/L HEPES, 10-7 mol/L dexamethasone, and antibiotics was used. Dishes were placed in a humidified incubator containing 50 mL/L CO2 and 950 mL/L O2 at 37°C.
Selection and passage of liver stem cells: Three days after culture, the medium and suspended cells were discarded and replaced by conditioning selection medium. Pure liver stem cells were selected by this medium. The cells were then proliferated with a new proliferating culture system, which contained 5% cholestatic serum, 10% FBS (Gibco), 10 mmol/L nicotinamide (Sigma), 1 mmol/L 2-phosphate ascorbic (Sigma), 1 mg/mL galactose (Sigma), 30 &mgr;g/mL praline (Sigma), insulin/transferrin/selenite mixture (Gibco), 10 ng/mL epidermal growth factor (EGF; Pepro Tech) and 10 ng/mL hepatocyte growth factor (HGF; Pepro Tech). When the cells developed into the proliferating stage, 10 ng/mL leukocyte inhibitory factor (LIF; Cytolab) was added to inhibit the differentiation of the cells. Passaging was carried out when the cells overlapped in the plate.
Differentiation of liver stem cells: Each passage of the stem cells was cultured in the differentiating system (i.e. the above culture system containing 20 ng/mL EGF, 25 ng/mL HGF, 1% DMSO (Sigma) and 20 ng/mL interleukin (IL)-3 (Pepro Tech), and LIF was discarded at this time. Differentiated cells were harvested.
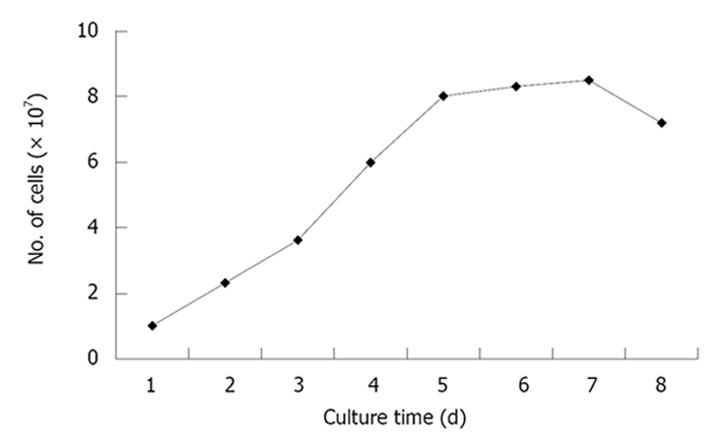
The passaged BDLSCs were digested and re-cultured on 24-well dishes at a concentration of 1 × 107/L. The mean number of cells in every three wells were counted daily for 8 d. The cell growth curve was then drawn according to these numbers.
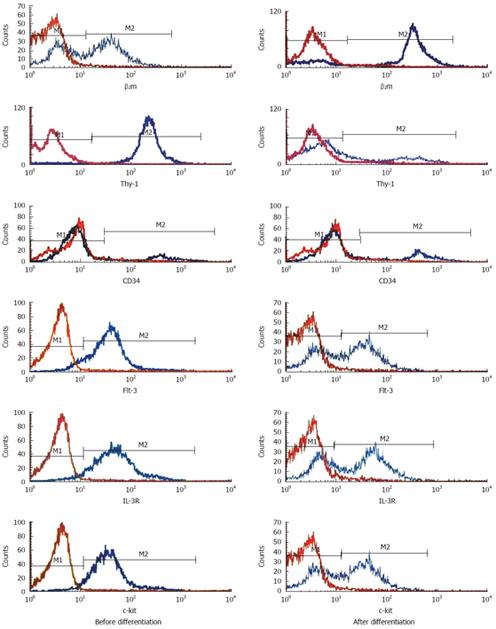
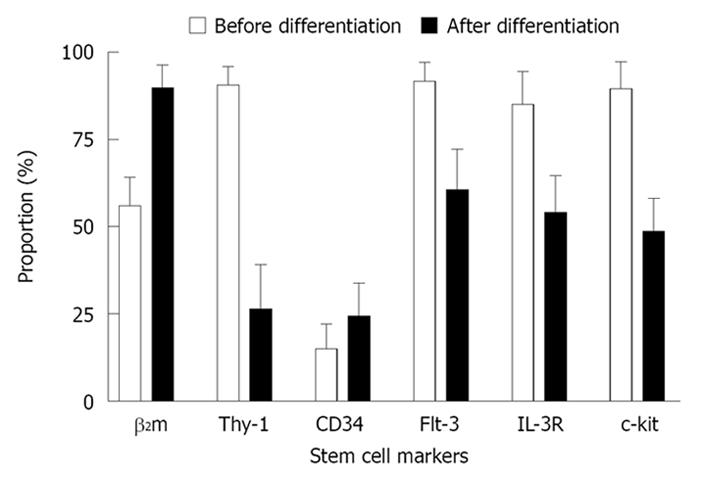
After each passage, the stem cells and the differentiated cells were digested to prepare single-cell suspensions. The cell surface markers beta-2 microglobulin (β2m), Thy-1, CD34, Flt-3, c-kit and IL-3R were detected with cytometry. Each marker was detected six times in every passage, and mean values were determined. The stability of stem cell surface marker expression was determined, and the expression before and after differentiation was compared.
Immunohistochemistry: Stem cells of each passage were cultured in the differentiating system in six-well dishes with cover glasses. When hepatocyte-like cells came into being, cover glasses and the differentiated BDLSCs were removed and fixed for immumohistochemistry. The primary antibodies were goat anti-rat albumin, alpha-fetoprotein (AFP), and cytokeratin -8/18 (CK8/18) polyclonal antibodies. (see our previous study)[4].
Electron microscopy: Culture dishes of differentiated cells were washed with PBS, and fixed in glutaraldehyde for 48 h. After fixation, cells were curetted and centrifuged to form aggregates. After post-fixation in osmium tetroxide, cells were dehydrated in graded alcohols and embedded in low-viscosity epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed under an electron microscope (see our previous study)[4].
Reverse transcription polymerase chain reaction (RT-PCR): Total RNA was extracted from the differentiated cells and mRNA transcription of hepatocyte nuclear factor (HNF)-1α, HNF-3β, CK18, CK19, albumin, AFP, transthyretin (TTR) and cytochrome P450-2b1 (CYP2b1) were detected by RT-PCR (see our previous study)[4].
Periodic acid-Schiff (PAS) staining for glycogen and urea assay of the differentiated cells were also conducted to confirm their hepatocyte-like function (see our previous study)[4].
Data were presented as mean ± SD. All the data were analyzed using the SPSS statistical package 13.0. Differences in means were tested by the unpaired Student’s t test. All tests were considered statistically significant at P < 0.05.
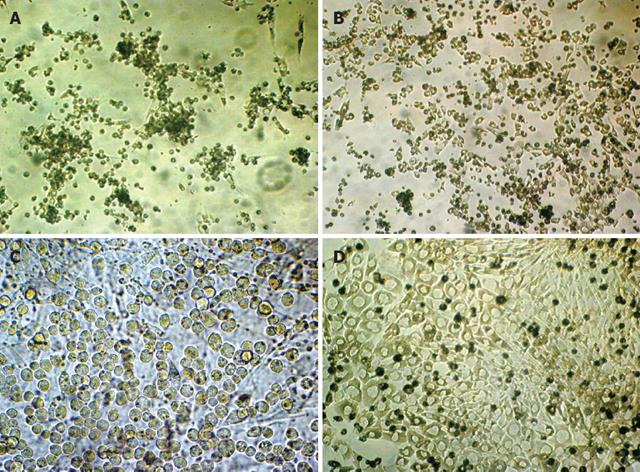
During the first 3 d, many colonies appeared in the conditioning cholestatic serum. These colonies were composed of small, undifferentiated cells in the center, and epithelioid cells at the periphery (Figure 1A). After replacing with the proliferating system, the colonies enlarged, and the cells proliferated rapidly in about 4 d (Figure 1B). With the addition of LIF, mature differentiation was inhibited, and colonies of small round cells with large nuclei, little endochylema and high nuclear-to-cytoplasmic ratio appeared (Figure 1C). The colonies maintained the ability of proliferation and passage was required in 5-7 d. The original aim of six passages could be achieved. After six passages, however, the proliferation was difficult to maintaine and fibroblast-like cells appeared. After replacing with the differentiating system, hepatocyte-like colony-forming units (H-CFUs) started to appear. The H-CFUs were composed of small, undifferentiated cells in the center, and large cells with regular multilateral contours, low nuclear-to-cytoplasmic ratio, and single round nuclei at the periphery. The differentiated cells formed cords or trabeculae that resembled the hepatocyte cords in hepatic lobules (Figure 1D).
The curve showed that the number of cells increased as the culture time passed, and rapid proliferation appeared from day 2 to day 5 (Figure 2).
Flow cytometry detecting the cell surface markers of each passage showed that the undifferentiating cells were relatively stable β2mlow/Thy-1+/CD34low/c-kit+ cells. The markers changed after differentiation, with significant differences (P < 0.05) in all of the detected markers except CD34 (Figures 3 and 4).
The differentiated cells from each passage were analyzed for biochemical evidence of hepatocytic differentiation, in order to confirm their characteristics. Just as in our previous study[4], immunohistochemistry was performed on differentiated cells grown on cover glasses to determine the presence of albumin, AFP and CK8/18, the characteristic proteins expressed during hepatocyte development, which revealed diffuse cytoplasmic staining for these proteins. RT-PCR further confirmed the hepatocytic characteristics of differentiated cells as the results showed that there were mRNA transcripts of HNF-1α, HNF-3β, albumin, AFP, CK-18, CK-19, TTR, and CYP2b1, all of which were hepatocyte specific.
Ultrastructurally, the differentiated cells were rich in endoplasmic reticulum and ribosomes and contained abundant ellipsoid mitochondria, which were typical features of adult hepatocytes.
We also found glycogen storage in the cytoplasm of hepatocyte-like differentiated cells by PAS staining, and urea production and secretion by urea assay of the culture medium[4].
Bone-marrow-derived stem cells are able to transdifferentiate into hepatic cells as shown in cross-sex and cross-strain bone marrow and whole liver transplantation experiments[5]. Great interest has been aroused in the identification and isolation of BDLSCs[6]. However, the characteristic surface markers of BDLSCs and their pedigrees in the derivation of bone marrow stem cells remain obscure[7]. It is difficult to identify and sort particular cells by immunological methods, such as fluorescence-activated and magnetic-activated cell sorting[89]. Furthermore, the sorting of stem cells with complicated surface markers is difficult[10]. We developed a conditioning culture system to solve this problem. Within such a system, only BDLSCs could survive, while other cells could not, therefore, it was possible to purify these specific stem cells[4].
However, the passage of BDLSCs is still a challenge, which has hindered the proliferation of the cells. No report of successful passage has been published. In our experiments, we found that the key to passage of the stem cells was to isolate purified stem cells. We were able to harvest pure BDLSC colonies with our selection system. After that, a proliferating system that contained all the nutrients required for the proliferation of liver stem cells was introduced to culture the cells[1112]. This system contained all the known conditions required for the proliferation of oval cells[13], with low concentrations of HGF and EGF and a mixture of FBS and cholestatic serum, so as to maintain the characteristics of the liver stem cells. At the same time, for stem cell proliferation, differentiation must be prevented. We therefore provided LIF, a factor known as a strong inhibitor of stem cell differentiation[14]. When replaced with the proliferating system on day 4, rapid proliferation occurred and the cells maintained their undifferentiated state, under the action of LIF. To harvest the differentiated cells, LIF must be discarded, and the concentration of EGF and HGF must be higher. Under the conditions that contained the differentiating factors, hepatocyte-like cells appeared from each passage of the stem cells. The morphology and phenotypic markers manifested in the differentiated cells were similar to those of liver stem cells. These cells expressed markers of embryonic hepatocytes (AFP, albumin and CK18), biliary cells (CK19), hepatocyte functional proteins (TTR and CYP2b1), and hepatocyte nuclear factors (HNF-1α and HNF-3β). To confirm that the differentiated cells had functional characteristics of hepatocytes, we tested the glycogen and urea synthesis functions of the cells, and demonstrated that the cells possessed hepatocyte-like functions. These all proved that each passage of the stem cells can differentiate into hepatocyte-like cells. We also detected the stability of each passage of the stem cells with flow cytometry, and the results showed that the proliferation was stable. In addition, the increase in β2m expression on the differentiated cells demonstrated their maturation because β2m is only expressed on mature cells. However, we had difficulty in maintaining the proliferation after six passages. The emergence of fibroblast-like cells became inevitable. The explanation might be that the proliferation of adult stem cells, unlike tumor cells, was limited, and that new factors or a network similar to the liver tissue were required for further proliferation.
In conclusion, we demonstrated that BDLSCs could be selected from whole bone marrow cells using conditioning medium. The stem cells could also be proliferated for six passages and differentiated into hepatocyte-like cells. These methods not only provide a new and effective method for the isolation and purification of extrahepatic liver stem cells, but also provide a readily available alternate source of cells for clinical hepatocyte therapy.
Bone-marrow-derived liver stem cells (BDLSCs) were once a hot topic in the field of stem cell research because of their important therapeutic implications, but little progress has been made in recent years because of the difficulty of isolation and proliferation of this special cell population. The authors developed a culture system to isolate BDLSCs from bone marrow cells, from which they could culture pure BDLSCs in vitro. However, the passage of BDLSCs is still a challenge, which has hindered the proliferation of the cells.
Great interests has been aroused in the identification and isolation of liver stem cells from bone marrow cells. Several subsets of bone marrow cells have been found to have the potential to differentiate into hepatocytes, but no report of successful passage has been published.
This study provided a new method for BDLSC isolation and proliferation. With a careful designed culture system, BDLSCs can be purified in vitro and be passaged, which brings new hope to the clinical use of bone-marrow-derived stem cells.
BDLSCs can be selected directly from bone marrow cells, and pure BDLSCs can also be proliferated for six passages. The differentiated cells have hepatocyte-like phenotypes and functions. BDLSCs represent a new method to provide a readily available alternate source of cells for clinical hepatocyte therapy.
In this study, the authors used their original method to retrieve cells that are possibly BDLSCs. Then, they used fluorescence-activated cell sorting to determine the cells’ characteristics before and after differentiation. This is an interesting and potentially important study, which suggests that bone-marrow-derived cells can be stimulated to expand and then differentiate into hepatocyte-like cells, which may possibly be used to treat liver disease.
| 1. | Vieyra DS, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65-69. |
| 2. | Inagaki Y, Higashiyama R, Okazaki I. Treatment strategy for liver fibrosis through recruitment and differentiation of bone marrow stem/progenitor cells. Hepatol Res. 2007;37:991-993. |
| 3. | Banas A, Quinn G, Yamamoto Y, Teratani T, Ochiya T. “Stem cells into liver”--basic research and potential clinical applications. Adv Exp Med Biol. 2006;585:3-17. |
| 4. | Cai YF, Zhen ZJ, Min J, Fang TL, Chu ZH, Chen JS. Selection, proliferation and differentiation of bone marrow-derived liver stem cells with a culture system containing cholestatic serum in vitro. World J Gastroenterol. 2004;10:3308-3312. |
| 5. | Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2-8. |
| 6. | Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215-230. |
| 7. | Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365-369. |
| 8. | Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230-238. |
| 9. | Okumoto K, Saito T, Hattori E, Ito JI, Adachi T, Takeda T, Sugahara K, Watanabe H, Saito K, Togashi H. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem Biophys Res Commun. 2003;304:691-695. |
| 10. | Popp FC, Piso P, Schlitt HJ, Dahlke MH. Therapeutic potential of bone marrow stem cells for liver diseases. Curr Stem Cell Res Ther. 2006;1:411-418. |
| 11. | Miyazaki M, Hardjo M, Masaka T, Tomiyama K, Mahmut N, Medina RJ, Niida A, Sonegawa H, Du G, Yong R. Isolation of a bone marrow-derived stem cell line with high proliferation potential and its application for preventing acute fatal liver failure. Stem Cells. 2007;25:2855-2863. |
| 12. | Shi XL, Qiu YD, Li Q, Xie T, Zhu ZH, Chen LL, Li L, Ding YT. Hepatocyte-like cells from directed differentiation of mouse bone marrow cells in vitro. Acta Pharmacol Sin. 2005;26:469-476. |
| 13. | Tirnitz-Parker JE, Tonkin JN, Knight B, Olynyk JK, Yeoh GC. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int J Biochem Cell Biol. 2007;39:2226-2239. |