Published online Mar 21, 2009. doi: 10.3748/wjg.15.1391
Revised: February 17, 2009
Accepted: February 24, 2009
Published online: March 21, 2009
We present the case history of a 3-year-old girl who was examined because of severe dystrophy. In the background, cow’s milk allergy was found, but her body weight was unchanged after eliminating milk from her diet. Other types of malabsorption were excluded. Based on nasal regurgitation and facial dysmorphisms, the possibility of DiGeorge syndrome was suspected and was confirmed by fluorescence in situ hybridization. The authors suggest a new feature associated with DiGeorge syndrome.
- Citation: Rózsai B, Kiss &, Csábi G, Czakó M, Decsi T. Severe dystrophy in DiGeorge syndrome. World J Gastroenterol 2009; 15(11): 1391-1393
- URL: https://www.wjgnet.com/1007-9327/full/v15/i11/1391.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1391
The main consequences of developmental abnormalities of the third and fourth branchial pouches are congenital heart defect, hypoparathyroidism, hypoplasia or aplasia of the thymus, velopharyngeal insufficiency and facial dysmorphism; the clinical picture is named DiGeorge syndrome. Apart from these major symptoms, many others including low muscle tone, short stature, hypothyroidism, kidney problems, developmental delay, psychiatric disorders and learning difficulties are often present[1]. In 40%-90% of children with DiGeorge syndrome feeding difficulties are also present[2]; nasal regurgitation is one of the first manifestations of a feeding abnormality in this group[2]. Eicher et al[3] convincingly demonstrated, by videofluorography, that dysmotility through the pharyngoesophageal segment is the major cause of dysphagia in this syndrome. Recently, failure of upper esophageal sphincter relaxation was also demonstrated by a videomanometric technique in three children with velocardiofacial syndrome[4]. Dysmotility of the upper gastrointestinal tract, velopharyngeal insufficiency and associated celiac disease[5] may all contribute to developmental delay in DiGeorge syndrome.
Here we present a case of an almost 3-year-old girl with cow’s milk allergy whose extreme dystrophy was not improved after eliminating milk from the diet, and in whom DiGeorge syndrome was later diagnosed as the background to the symptoms.
The girl was born after an uneventful pregnancy at the 38th week of gestation with a birthweight of 2950 g, and Apgar scores of 10-10 at 5 and 10 min. She was breast fed for 6 mo, her development was normal, and her body weight was 5500 g at the age of 6 mo. However, after finishing breast feeding and changing to follow-on formula, her body weight did not increase. In addition, episodes of infections with 38°C-38.5°C fever appeared, and several times, antibiotic treatments were initiated. Her mother reported that the infant sometimes had nasal regurgitation. No gross abdominal complaints were observed, and only moderate reflux was detected by ultrasonography. At 11 mo of age, routine laboratory tests and abdominal ultrasound were normal. An anti-endomysial antibody test, chloride sweat test, and stool cultures were negative. To exclude celiac-like lesions, a small bowel biopsy was performed, but normal bowel mucosa was found. With a background of dystrophy, cow’s milk and soy-protein allergy was diagnosed by specific immunoglobulin E (IgE) assay. The hypotonic musculature was explained as the result of dystrophy, and her creatine kinase level was only slightly elevated (276 U/L, normal range: < 200 U/L). Milk was removed from her diet and oral iron therapy was administered; however, her body weight did not increase. At 17 mo of age, reflux was no longer detectable, her appetite was normal, but still no weight gain was observed.
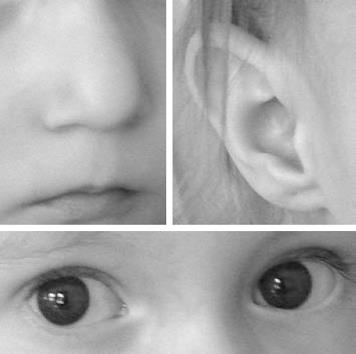
At 20 mo, the girl was admitted to our department for further examinations to resolve the cause of the severe dystrophy. Her body weight was 6000 g (< 3rd percentile) and her length was 76 cm (< 3rd percentile). In association with the signs of dystrophy, moderate psychomotoric retardation was seen and some minor anomalies were found during physical examination. These included ocular hypertelorism, micrognatia, flat nasal bridge, bulbous nasal tip, short philtrum, and slightly rotated ears (Figure 1). Laboratory tests including blood count, electrolytes, liver enzymes, pre-albumin, albumin, ammonia, iron and Ig levels, as well as the carbohydrate malabsorption test were normal. Serum amino acids showed a normal pattern. Otolaryngologic examination revealed dysfunctional soft palate movement. Echocardiography found a chorda tendinea in the left ventricle, which was thought to be a normal variant. Psychologic testing showed mental retardation (IQ: 61.6).
Based on facial dysmorphism, the nasal regurgitation seen earlier and the newly diagnosed velopharyngeal insufficiency, the possibility of DiGeorge syndrome was considered. Our suspicion was confirmed by fluorescence in situ hybridization analysis which showed microdeletion on the long arm of one of chromosome 22. We were unable to visualize the thymus either by X-ray examination, or by thoracic ultrasound. Flow cytometric analysis did not reveal immunodeficiency; the number of T lymphocytes was 3052/&mgr;L (normal range: 2100-6200/&mgr;L). A normal cytokine response occurred after simulating her lymphocytes with bacterial lipopolysaccharide. The ionized calcium, serum parathyroid hormone, thyroid stimulating hormone and vitamin D levels were in the normal range [1.22 mmol/L (normal: 1.1-1.3 mmol/L), 1.9 pmol/L (normal: 1.6-6.9 pmol/L), 2.96 mU/L (normal: 0.27-4.2 mU/L), 155 nmol/L (normal: 47.7-170 nmol/L), respectively]. The daily calorie intake calculated from the dietary record of the mother was about 800-900 kcal, therefore hypercalorization was recommended (1200-1300 kcal/d). Following administration of this diet, her body weight began to increase, but it still remained below the 3rd percentile line.
The frequency of upper airway infections increased and Candida was cultured from the stools, therefore, long-term antifungal therapy was started. She complained of indefinite pain of the upper limbs. At 2 years of age she was hospitalized several times because of pneumonia. At this time the serum total calcium level was decreased (1.8 mmol/L; normal: 2.1-2.4 mmol/L), so calcium-gluconicum and cholecalciferol were given in doses of 3 × 500 mg/d and 400 IU/d, respectively. At the next examination, the total calcium level was found to be decreased further (1.54 mmol/L), the ionized calcium level was also low (0.68 mmol/L), and hyperphosphatemia (2.33 mmol/L; normal: 1.0-2.0 mmol/L) and hypoparathyroidism (1.58 pmol/L) were found. Cholecalciferol was changed to calcitriol (1 × 0.25 &mgr;g/d). The total calcium level began to increase after a week of calcitriol administration (1.87 mmol/L ). Calcium homeostasis normalized following a month of calcitriol therapy.
The diagnosis of DiGeorge syndrome in the present case was rendered difficult by the apparent explanation of the failure to thrive as serologically verified cow’s milk allergy. Though our patient had neither atopic dermatitis nor recurrent episodes of obstructive bronchitis, which often accompany symptoms of cow’s milk allergy[6], cow’s milk-protein-specific IgE levels indicated cow’s milk allergy. However, diagnosing cow’s milk allergy and eliminating milk from the diet did not result in the expected weight gain. So cow’s milk allergy on its own did not explain the failure to thrive in our patient. Mention of nasal regurgitation provided important information, because this is a classic finding in infants with DiGeorge syndrome[2]. Detailed physical examination revealed minor anomalies (Figure 1) that fit well into the suspected diagnosis. A cardiac defect was admittedly absent in our patient; however, in about 20% of patients with DiGeorge syndrome there is no heart disease[1].
Dysmotility of the upper gastrointestinal tract is a well known complication in DiGeorge syndrome[2–4], which together with growth hormone deficiency, hypothyroidism and velopharyngeal insufficiency may lead to developmental delay and dystrophy. Digilio et al reported that 8.3% of their patients with DiGeorge syndrome had body weight below the 3rd percentile and all of them were younger than 5 years of age. They also presented a 2-year-old girl who had high level of anti-endomysial antibodies and in whom celiac disease was diagnosed after jejunal biopsy[5]. As far as we know our case is the first in which an association between cow’s milk allergy and DiGeorge syndrome is represented.
In summary, we recommend screening for DiGeorge syndrome in patients with classical diseases of the gastrointestinal tract if poor weight gain is also present. Nasal regurgitation during infancy is an important sign that suggests the possibility of DiGeorge syndrome.
| 1. | Goldmuntz E. DiGeorge syndrome: new insights. Clin Perinatol. 2005;32:963-978, ix-x. |
| 2. | Cuneo BF. 22q11.2 deletion syndrome: DiGeorge, velocardiofacial, and conotruncal anomaly face syndromes. Curr Opin Pediatr. 2001;13:465-472. |
| 3. | Eicher PS, McDonald-Mcginn DM, Fox CA, Driscoll DA, Emanuel BS, Zackai EH. Dysphagia in children with a 22q11.2 deletion: unusual pattern found on modified barium swallow. J Pediatr. 2000;137:158-164. |
| 4. | Rommel N, Davidson G, Cain T, Hebbard G, Omari T. Videomanometric evaluation of pharyngo-oesophageal dysmotility in children with velocardiofacial syndrome. J Pediatr Gastroenterol Nutr. 2008;46:87-91. |
| 5. | Digilio MC, Giannotti A, Castro M, Colistro F, Ferretti F, Marino B, Dallapiccola B. Screening for celiac disease in patients with deletion 22q11.2 (DiGeorge/velo-cardio-facial syndrome). Am J Med Genet A. 2003;121A:286-288. |
| 6. | Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, Staiano A, Dupont C. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007;92:902-908. |