Published online Mar 21, 2009. doi: 10.3748/wjg.15.1359
Revised: February 23, 2009
Accepted: March 2, 2009
Published online: March 21, 2009
AIM: To culture human pancreatic tissue obtained from small resection specimens as a pre-clinical model for examining virus-host interactions.
METHODS: Human pancreatic tissue samples (malignant and normal) were obtained from surgical specimens and processed immediately to tissue slices. Tissue slices were cultured ex vivo for 1-6 d in an incubator using 95% O2. Slices were subsequently analyzed for viability and morphology. In addition the slices were incubated with different viral vectors expressing the reporter genes GFP or DsRed. Expression of these reporter genes was measured at 72 h after infection.
RESULTS: With the Krumdieck tissue slicer, uniform slices could be generated from pancreatic tissue but only upon embedding the tissue in 3% low melting agarose. Immunohistological examination showed the presence of all pancreatic cell types. Pancreatic normal and cancer tissue slices could be cultured for up to 6 d, while retaining viability and a moderate to good morphology. Reporter gene expression indicated that the slices could be infected and transduced efficiently by adenoviral vectors and by adeno associated viral vectors, whereas transduction with lentiviral vectors was limited. For the adenoviral vector, the transduction seemed limited to the peripheral layers of the explants.
CONCLUSION: The presented system allows reproducible processing of minimal amounts of pancreatic tissue into slices uniform in size, suitable for pre-clinical evaluation of gene therapy vectors.
-
Citation: Geer MAV, Kuhlmann KF, Bakker CT, Kate FJT, Elferink RPO, Bosma PJ.
Ex-vivo evaluation of gene therapy vectors in human pancreatic (cancer) tissue slices. World J Gastroenterol 2009; 15(11): 1359-1366 - URL: https://www.wjgnet.com/1007-9327/full/v15/i11/1359.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1359
Pancreatic cancer is a devastating disease. The mortality rate of this cancer almost equals its incidence rate. Genetic alterations in pancreatic cancer, as reviewed by Saif et al[1], result in an aggressive cancer that is resistant to medical intervention. Surgical resection of pancreatic adenocarcinoma remains the cornerstone of treatment but is only available for a small minority of patients. Even after surgical intervention prognosis remains poor, since median and 5-year survival after resection are 7.5 mo and 8%, respectively[2]. A recent meta-analysis suggests that gemcitabine-based chemotherapy seems to improve overall survival of patients with advanced and metastatic disease to some extent[3]. However, even with this treatment the prognosis continues to be poor. New therapies, such as viral-vector-mediated gene therapy, are needed to improve the survival of patients with pancreatic cancer.
Adenoviral vectors are used most frequently in gene therapy strategies aimed to treat solid cancers. Important advantages of this vector are its capability to infect and transduce dividing and non-dividing cells and that it can be propagated to high titers. Initially, non-replicating adenoviral vectors were used to introduce expression of a cytotoxic or a pro-drug activating gene into cancer cells. This so-called suicide gene therapy approach did show efficacy in pre-clinical cancer models. However, in the subsequent phase 1 clinical trials, no therapeutic effect was observed due to limited spread of the viral vector in the tumors. To improve the efficacy and the spread, adenoviral vectors were developed that were able to replicate in cancer cells but not in normal cells. These conditional replicating adenoviral vectors (CRAds) replicate specifically, in tumor cells and kill them as result of their lytic cycle. Upon replication a large amount of virus progeny will be generated in a tumor and infect other cancer cells. In pre-clinical models, these CRAds were more efficient than the non-replicating vectors. At least six different CRAds have subsequently been evaluated in clinical trials but only a minority of these studies suggested that these vectors could be effective[4]. The first engineered oncolytic adenovirus that was implemented in a clinical setting was Onyx-015, which replicates specifically in p53-deficient tumor cells. Preclinical studies had shown that Onyx-015 efficiently kills human cancer cells in subcutaneous tumors in nude mice[56]. Subsequent clinical studies showed that ONYX lacked efficacy as a single anti-tumor agent[7]. Also, in phase I/II trials for pancreatic cancer, intra-tumoral injection of Onyx-015 lacked efficacy[89]. Further studies showed that for these replicating adenoviral vectors, the transduction of cancer cells in vivo also appeared to be the limiting factor in treating patients.
Low expression of the coxsackie- and adenovirus receptor (CAR), via which adenovirus normally enters cells, was subsequently shown to cause the poor transduction of pancreatic cancer in vivo[10]. To overcome this problem several strategies emerged to increase pancreatic cancer transduction by circumventing CAR-mediated entry[11]. Insertion of the Arg-Gly-Asp (RGD) peptide motif in the fiber knob of adenovirus, for instance, enhances infectivity of pancreatic tumors in a mouse model[12]. All such studies however rely on the use of human pancreatic cancer cells growing subcutaneously in a nude mouse model. The discrepancies in efficacy of novel treatments seen in these preclinical studies and in subsequent clinical trials demonstrates that this is not an ideal model. For instance, these mouse models do not assess the cellular heterogeneity of solid tumors such as pancreatic cancers. In addition, they do not accumulate extracellular matrix, which occurs in human tumors. Finally, due to the lack of normal human pancreas cells, these models are not suitable for demonstrating increased tumor specificity of the retargeted adenoviral vector. Therefore new models are needed that more closely resemble the in vivo situation in patients. Tissue explants seem a good alternative in this respect. In these explants, the interaction between heterogeneous cell types comprising normal and tumor tissue is retained. In addition, these explants do contain extracellular matrix. Therefore, tissue explants seem a suitable model to study transduction efficiency in a setting more representative of the in vivo situation.
Several culture systems are available that allow the preservation of live pancreas tissue taken directly from patient specimens. Hoem et al[13] cultured normal exocrine pancreatic cells, derived from pancreaticoduodenectomy specimens, as spheroids for at least 6 wk. A similar method was used for culturing pancreatic adenocarcinomas[14]. Both groups manually dissected tumor specimens to small fragments or cubes. Manual dissection of fresh tissue, however, causes physical stress and generates fragments of variable size and shape. This will influence the transduction by viral vectors because it affects the viability of the tissue and surface/volume ratio. To generate tissue slices with a more consistent size and shape, we decided to use an automatic tissue slicer. These slicers are widely used to generate precision-cut organ slices for biotransformation research. Slices can be prepared from several organs including liver, lung, kidney, colon and intestine. They closely resemble the architecture of the original organ, which makes this technique a powerful instrument to perform toxicity and drug-metabolizing studies[15]. Recently, the ultra-thin slices also proved to be a representative model for ex vivo evaluation of the transduction efficiency of adenoviral vectors[1617].
Precision-cut slicing of human pancreatic tissue was never reported. The aim of the present study was to evaluate the use of slices to test adenoviral-vector-mediated gene therapy for pancreatic cancer. We performed tissue slicing and subsequent culture of human pancreatic tissue slices and examined viability and morphology over time of normal and malignant pancreatic explants. Since we show that patient explants derived from minimal amounts of tissue are viable for up to 6 d with preservation of morphology, these slices appear to be a good model for studying viral-vector-mediated gene therapy and also seem suitable for pre-clinical testing of novel drugs to treat pancreatic disease.
MD4000 Krumdieck tissue slicer was from Alabama Research & Development, Munford, Alabama; L-glutamine, penicillin, streptomycin, amphotericin were from Cambrex Bio Science, Walkersville, Maryland; insulin, transferrin and selenium solution, MEM vitamins and MEM amino acids were from Invitrogen; the Innova 4300 incubator was from New Brunswick Scientific Co, Edison, New Jersey; the NOVOstar fluorometer was from BMG Lab Technologies, Offenburg, Germany; WST-1 assay was from Roche, Almere, Netherlands; mouse anti-GFP JL-8 was from Clontech, Palo Alto, CA. The Powervision system was from ImmunoLogic, Duiven, The Netherlands; the biotinylated rabbit anti-mouse immunoglobulins and streptavidin–horseradish peroxidase conjugate were from DAKO Netherlands.
Virus generation and propagation: Plasmids encoding for E1-, E3-deleted adenovirus vectors were modified by using pAdHM15[18]. pAdHM15 was digested with PI-Sce and Ceu-I to insert the reporter genes enhanced green fluorescent protein (GFP) and DsRed under control of the CMV promoter[10]. Recombinant adenoviral vectors were generated by transfection of HEK 293 cells with PacI-linearized Ad-GFP and Ad-dsRED. Virus was purified and concentrated by performing two cesium chloride gradients according to standard protocols. Virus preparations were dialyzed two times against 1 L of PBS. After the second dialysis, glycerol was added to a final concentration of 10% (v/v) and virus preps were aliquoted, and stored at -80°C. The number of genomic copies was determined by quantitative real-time polymerase chain reaction by using the primers against hexon DNA[19].
Lentivirus-CMV-GFP was constructed as described before, by transfection of 293T cells using a calcium phosphate method, concentrated by ultracentrifugation, and titrated on HeLa cells[20]. AAV2-CMV-GFP was constructed and titers were determined as described previously[21].
Primary tissue slices: Fresh human pancreatic specimens were obtained from patients undergoing a pancreaticoduodenectomy for pancreatic head tumors (pancreatic cancer slices) and carcinomas of the bile duct or ampulla of Vater (normal pancreas slices). Tumor specimens were embedded in 3% low melting point agarose/PBS before cutting in the MD4000 Krumdieck tissue slicer, to produce slices with a thickness of approximately 250 &mgr;m (estimated 2.5 × 105 cells), while submerged in oxygenated ice-cold Krebs. Slices were incubated in 1 mL DMEM including L-glutamine (2 mmol/L), penicillin (100 U/mL), streptomycin (100 &mgr;g/mL) and amphotericin (Fungizone 2.5 &mgr;g/mL) with or without insulin, transferrin and selenium (ITS), and 20 mmol/L HEPES pH 7.4. After 1 h, medium was replaced and slices were infected with 1 × 108 or 5 × 108 genomic copies Ad-GFP and/or Ad-DsRED, or with 1.0 × 106 transducing units of lentivirus-CMV-GFP or with 1.2 × 1010 genomic copies AAV-2-CMV-GFP. All experiments were performed in triplicate and were repeated at least three times.
Tissue culture plates were placed in an Innova 4300 incubator that was humidified and gassed with 95% O2 and continuously shaken back and forth (90 times/min) at 37°C. Virus was removed and medium was refreshed after 36 h. Slices were harvested at several time points and lysed overnight at 4°C in 60 &mgr;L RIPA buffer (25 mmol/L Tris HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). After one round of freeze/thawing, cellular debris was removed by centrifugation. Total fluorescence of GFP and dsRED in supernatant was measured with a NOVOstar fluorometer. The filter settings were as follows: GFP excitation at 485 nm, GFP emission at 520 nm, DsRed excitation at 550 nm, DsRed emission at 580 nm. Several slices were fixed in 4% paraformaldehyde.
WST-1 and amylase secretion assay: Viability of tissue explants was determined with the WST-1 assay according to the protocol of Roche. Amylase activity in culture medium was determined using an enzyme-based colorimetric assay on a P800 Modular Roche Diagnostics apparatus.
Histology and immunohistology: Tissue slices were fixed in 4% paraformaldehyde, embedded in paraplast and 7-&mgr;m sections were made. Hematoxylin and eosin staining was performed according to standard protocols. Sections were assessed for viability and morphological characteristics. Paraffin-embedded sections were used for immunohistological detection of GFP as reported earlier[21].
Several studies underline the relevance of tissue slice technology for experiments involving organ culture. Solid organs such as liver can be sliced directly with a tissue slicer. For human pancreas, this was not possible because it is too soft. We showed that this problem could be solved by embedding small (approximately 5 mm) pancreatic fragments in cylinders of 3% low melting agarose. The resulting core was solid enough for processing in a tissue slicer. Various slicing systems are available commercially.
Initially, we used the Brendel/Vitron tissue slicer. However, we found the pancreatic slices to vary significantly in viability, integrity and size. Therefore, these preparations contained a large percentage of tissue fragments not suitable for further experiments. Furthermore, operating this slicer requires both hands, which compromised the retention of aseptic conditions in the biosafety flow cabinet. Therefore, we switched to the fully automated Krumdieck tissue slicer. With this slicer, many more uniform slices with considerably less damage to the fragile pancreatic tissue were obtained.
The study of Wang et al[14] suggests that supplement-enriched culture medium extends tissue survival. We therefore added ITS, amino acids and vitamins to the culture medium. The oxygen tension varies between culture systems reported[15]. We decided to gas the incubator with 95% O2 to ascertain proper diffusion of oxygen to the inner cell layers.
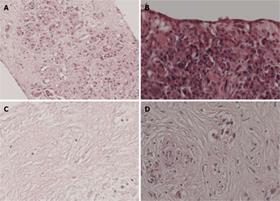
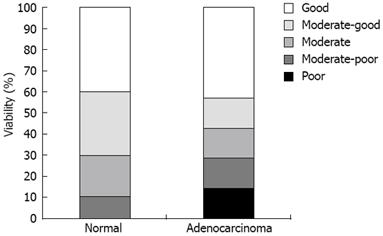
To examine the quality over time of cultured pancreatic slices, we first assessed viability by histology. Parameters for viability included cell swelling, necrosis and nuclear pyknosis. We included slices from 10 normal pancreas specimens and six pancreatic adenocarcinomas for examination at 3 d after slicing. In normal pancreas, we were able to distinguish all cell types including exocrine cells, ductal cells, islets of Langerhans, and nerve cells. Figure 1A shows a normal pancreas tissue slice at day 3. We observed that a cell sheet consisting of squamous epithelial-like cells (Figure 1B) covered several tissue slices. Tissue slices of poor viability were characterized by complete disappearance of viable cells (Figure 1C). Figure 1D shows poorly differentiated adenocarcinoma of good quality. Some pancreatic cancers were accompanied by stromal tissue consisting of fibroblasts and connective tissue. All 16 slices were scored for viability. For normal pancreas, 70% of the slices had moderate to good viability, while, for pancreatic cancer specimens, this was 57% (Figure 2). Not surprisingly, keeping the time between resection and start of tissue culture as short as possible improved the viability of the slices.
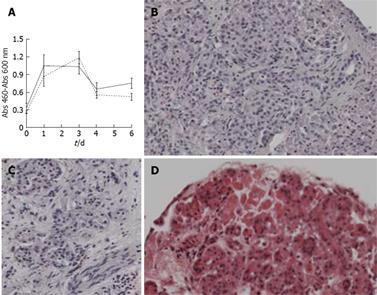
To examine the effect of medium formulation on the viability of the slices from normal human pancreas, we performed a WST-1 assay. Tissue slices were cultured either in normal culture medium or in medium supplemented with ITS, vitamins and amino acids. The WST-1 reaction was performed at day 0, 1, 3, 4 and 6. WST-1 measurements showed an increase in activity after 1 d of culture, which remained constant up to day 3 and then showed a gradual decline at day 4 and 6 (Figure 3A). Addition of ITS, vitamins and amino acids did not significantly improve viability of the tissue slices. After 6 d in culture, these slices were subsequently examined for morphology. Figure 3B and C shows a representative overview of normal pancreas of good viability, including Langerhans cells and nerve cells. We cultured two additional sets of normal pancreas slices for 6 d and performed histological staining. We noted moderate to good viability in one and moderate to poor quality, in the second. In slices with poor quality the deterioration of the tissue started in the peripheral cell layers. Even in these slices, moderate quality was maintained in the center (Figure 3D). Based on these histological comparisons we concluded that pancreatic slices retain moderate to good morphology for at least 3 d in culture. The WST-1 conversion at day 6 and moderate to good morphology seen in several slices suggests that these cultured pancreatic tissue slices remain viable for even longer periods.
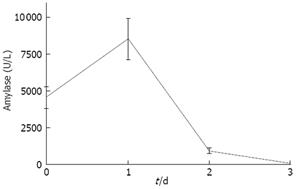
In addition to viability and morphology, we tested functionality of pancreas slices by determining amylase activity in the medium. Medium was analyzed during three consecutive days following explanting of tissue. As shown in Figure 4, amylase secretion is maximal at day 1 and declined below detection level at day 3. This secretion of amylase suggests that the slices are not just viable and retain the correct morphology, but that they are also functional at least up to day 2.
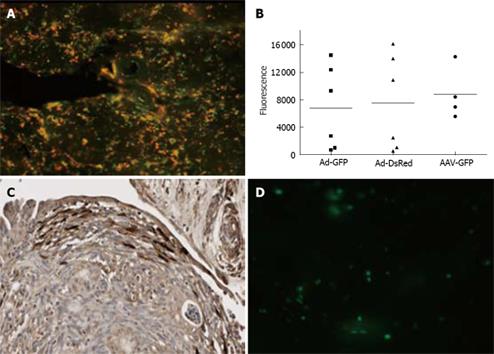
The possibility of culturing slices for up to 3 d renders this system suitable for studying the transduction of pancreatic tissue by adenoviral vectors that require 48 to 72 h to reach maximal gene expression levels. We examined whether pancreatic tissue slices produce detectable levels of two different reporter proteins after transduction with adenoviral vectors. We chose to test the transduction in these slices with an adenovirus vector expressing GFP and with one expressing dsRed. Since both are Ad-5 vectors and will display their native tropism, we expect they should have overlapping transduction patterns. Figure 5A shows diffuse expression of GFP and DsRed upon transduction with these vectors, and as expected, the expression patterns of both reporter genes largely overlap. To quantify the expression levels of both reporter genes, the slices were lysed and used for fluorometric detection of GFP and ds-Red in the NOVOstar reader. Infection of normal pancreas and cancer tissues with 1.0 × 108 and 5.0 × 108 gc/slice, respectively, resulted in clearly detectable measurements of both reporter genes at 72 h after transduction (Figure 5B). Lower amounts of virus or measurements at earlier time points resulted in expression levels too low to measure accurately. As shown in Figure 5B, the expression of both reporter genes varies significantly between slices. These variations most likely reflect differences in viability, in size and in tissue composition between slices. Importantly, by dividing the remaining fluorescence values of GFP by DsRed we obtained an average GFP to DsRED ratio of 0.9. In addition to the overlapping expression pattern, this constant ratio between both reporter genes also indicates that one of these vectors can be used as internal control for slice quality. For instance, in case of co-transduction with a tropism modified vector, the GFP/DsRed ratio will reflect the improved transduction efficiency of the targeted vector in comparison to the wild type adenovirus.
The transduction of the slices by adenovirus is not homogeneous. As shown by the immunohistological staining in Figure 5C, transduction seemed limited to the peripheral layers of the explants. This non-homogeneous transduction pattern underscores the importance of co-transduction with an internal control when using this system to test the effect of retargeting of gene therapy vectors.
The possibility of culturing pancreatic slices for up to 3 d also seems long enough to study the transduction with other types of viral vectors such as lentiviral vectors and adeno associated virus (AAV). To explore this possibility we incubated normal pancreas with adeno-associated virus serotype 2-GFP (AAV2-GFP) or with lentivirus-GFP. Upon incubation with AAV-GFP, the fluorescence levels were comparable to those obtained with Ad-GFP (Figure 5B). In contrast, fluorescence was below detection limit when using lentivirus-GFP. As shown in Figure 5D, only a few cells were transduced by lentiviral vectors and expressed GFP. This poor transduction by lentiviral vectors may have been due to the vigorous shaking of the slices in combination with a low affinity of this vector for this tissue. Another possible cause is the fragility of lentiviral vectors. Production of proteases and/or lipases by these slices will affect lentiviral vector integrity and reduce transduction.
The limited intervention options for advanced and metastasized pancreatic cancer require new treatment modalities such as gene therapy. The preclinical adenovirus-mediated gene therapy study models primarily rely on cultured cells and the xenograft nude mouse model. Both remain a poor representation of in vivo pancreatic cancer. Pancreatic cell lines acquire new genetic defects upon multiple passages in vitro and after subcutaneous injection in vivo[22]. Moreover, xenograft tumors lack the natural interaction between tumor cells, stromal cells, normal cells and extracellular matrix. This is an important drawback since anatomical barriers such as fibrosis are a major hurdle for adenovirus spreading through the tumor[23].
For these reasons, primary human tissue specimens seem more comparable to the in vivo situation and may provide a better prediction of virus-host interactions.
We studied the tissue slice system for culturing ex vivo pancreatic explants. Other groups have already reported slice culture of various organs from different species[15–1724]. Here, we demonstrated that this technique is also feasible for pancreatic tissue using the Krumdieck tissue slicer. Independently of the medium formulation, normal pancreas was viable for up to 6 d. Since the inner cell layers seemed more viable than the peripheral layers, oxygen and nutrient diffusion through the slices does not seem limiting. Microscopic examination of normal slices revealed an epithelial-like cell sheet surrounding the explants. This is in accordance with the findings of Hoem et al, who cultured ex vivo pancreas for up to 6 wk[13]. They hypothesized that these cells have a ductal or acinar cell origin, possibly resulting from transdifferentiation caused by prolonged culturing. Others showed that this so-called acinar-ductal transdifferentiation in vitro indeed depends on medium formulation[25]. The loss of ductal and acinar cells from these explants may explain the decrease of amylase secretion by the slices over time.
We also showed that pancreatic adenocarcinoma explants can be cultured while retaining moderate to good viability and morphology. Resection specimens are small and the amount of tissue available for research purposes therefore is very limited. Manual dissection to fragments led to inhomogeneous samples that could not be used for further study. To optimize the yield of slices, we therefore used an automatic tissue slicer. The size of the resection specimens varied, and to be able to generate uniform slices, the specimens were included in low melting agarose. Upon stabilizing the tumor cubes in this manner, a significant number of slices could be obtained from limited amounts of tissue. This method does result in slices comparable in size and viability, with minimal loss of tissue. As such, this method seems superior to manual processing that resulted in slices with a high degree of heterogeneity and limited survival of explanted tissue[14]. Furthermore, with this technique, several slices can be obtained from a small resection specimen. This enabled us to compare different viral vectors and experimental conditions in the resection specimens derived from each patient.
We were able to culture these slices for at least 3 d with good morphology. This period is sufficiently long to study the transduction efficiency since most viral vectors need between 48 and 72 h for optimal expression. We showed that adenovirus infects and transduces pancreatic slices efficiently. In addition, we showed that simultaneous transduction of slices with a control and experimental virus expressing different reporter genes provides an elegant procedure to determine adenoviral transduction efficacy while correcting for variability in tissue viability and composition. In this respect, this system seems highly suitable for determining the efficacy of adenoviral vectors that target pancreatic cancer cells. Transduction experiments with such infectivity-enhanced adenoviral vectors in slices from normal tissue and tumor tissue will indicate their targeting specificity. Others have already shown that slices that maintain viability for at least 3 d are suitable for replication of CRAds[1724]. Therefore, our tissue explant system seems suitable to test the transduction efficacy of CRAds, and more especially, to investigate the increased efficacy of infectivity-enhanced CRAds. However, an optimal comparison of actual oncolytic potency of CRAds requires several rounds of replication and therefore a prolonged culture time. For these investigations, patient-derived multicellular cancer spheroids seem more suitable[2627].
In addition to adenoviral transduction, these slices were efficiently transduced by AAV-2 but poorly by lentiviral vectors. These two vectors have been shown to be suitable for correction of inherited disorders in pre-clinical models. Based on the transduction seen here, these slices can be used to investigate whether these vectors can be targeted; for instance, to the islet cells with the aim of providing correction for diabetes[28].
In conclusion, we have developed a system that allows processing of minimal amounts of pancreatic tissue from resection specimens. The generated thin, homogenous and viable tissue slices retain their morphology for at least 3 d. Furthermore, with this method, a sufficient number of slices can be obtained from minimal amounts of tissue, enabling the comparison of several experimental conditions within a single tumor specimen.
We acknowledge Jan Dekker for his technical assistance and professor DJ Gouma for providing resection material.
Pancreatic cancer is a devastating disease for which at present no therapy is available. Gene therapy was shown to be effective in preclinical animal models and was expected to improve the poor prognosis for patients suffering from pancreatic cancer. However, in patients, these methods lacked efficacy due to poor transduction of cancer cells. Therefore, other models more comparable to the situation in patients are essential for predicting the clinical efficiency of novel therapies such as gene therapy.
To circumvent the low coxsackie- and adenovirus receptor expression in human cancers in patients, retargeting of adenovirus to receptors highly expressed on human cancers is an essential step to improve their clinical efficacy.
Available animal models for pancreatic cancer do not reliably predict the clinical efficacy of retargeted adenoviral vectors. In this study, we present a tissue slice model more representative of the situation in patients as a model to predict the clinical efficacy of retargeted adenoviral vectors.
This tissue slice model is suitable for determining the efficiency of viral vectors retargeted to pancreatic cancer. In addition, this system can be used for pre-clinical ex-vivo studies of novel drugs to treat pancreas cancer.
This was a well-performed study in an area of interest to gastroenterologists and pancreatologists in particular.
| 1. | Saif MW, Karapanagiotou L, Syrigos K. Genetic alterations in pancreatic cancer. World J Gastroenterol. 2007;13:4423-4430. |
| 2. | Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549-558. |
| 3. | Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607-2615. |
| 4. | Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429-432. |
| 5. | Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639-645. |
| 6. | Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134-1139. |
| 7. | Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8:89-98. |
| 8. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. |
| 9. | Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308-315. |
| 10. | Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, Li H, Parameshwar M, Vickers SM, Jaffee EM. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969-976. |
| 11. | Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034-1044. |
| 12. | Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203-1218. |
| 13. | Hoem D, Dalen H, Andrén-Sandberg A, Höstmark J. Nonadhesive organ culture of human exocrine pancreatic cells with their stroma. Pancreas. 2002;25:71-77. |
| 14. | Wang Y, Thorne S, Hannock J, Francis J, Au T, Reid T, Lemoine N, Kirn D, Halldén G. A novel assay to assess primary human cancer infectibility by replication-selective oncolytic adenoviruses. Clin Cancer Res. 2005;11:351-360. |
| 15. | de Kanter R, Monshouwer M, Meijer DK, Groothuis GM. Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr Drug Metab. 2002;3:39-59. |
| 16. | Marsman WA, Buskens CJ, Wesseling JG, Offerhaus GJ, Bergman JJ, Tytgat GN, van Lanschot JJ, Bosma PJ. Gene therapy for esophageal carcinoma: the use of an explant model to test adenoviral vectors ex vivo. Cancer Gene Ther. 2004;11:289-296. |
| 17. | Kirby TO, Rivera A, Rein D, Wang M, Ulasov I, Breidenbach M, Kataram M, Contreras JL, Krumdieck C, Yamamoto M. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697-8703. |
| 18. | Mizuguchi H, Koizumi N, Hosono T, Utoguchi N, Watanabe Y, Kay MA, Hayakawa T. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001;8:730-735. |
| 19. | Ma L, Bluyssen HA, De Raeymaeker M, Laurysens V, van der Beek N, Pavliska H, van Zonneveld AJ, Tomme P, van Es HH. Rapid determination of adenoviral vector titers by quantitative real-time PCR. J Virol Methods. 2001;93:181-188. |
| 20. | Seppen J, Rijnberg M, Cooreman MP, Oude Elferink RP. Lentiviral vectors for efficient transduction of isolated primary quiescent hepatocytes. J Hepatol. 2002;36:459-465. |
| 21. | Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, de Waart R, Twisk J, Bosma P. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther. 2006;13:1085-1092. |
| 22. | Reyes G, Villanueva A, García C, Sancho FJ, Piulats J, Lluís F, Capellá G. Orthotopic xenografts of human pancreatic carcinomas acquire genetic aberrations during dissemination in nude mice. Cancer Res. 1996;56:5713-5719. |
| 23. | Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss HP. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520-1535. |
| 24. | Rots MG, Elferink MG, Gommans WM, Oosterhuis D, Schalk JA, Curiel DT, Olinga P, Haisma HJ, Groothuis GM. An ex vivo human model system to evaluate specificity of replicating and non-replicating gene therapy agents. J Gene Med. 2006;8:35-41. |
| 25. | Sphyris N, Logsdon CD, Harrison DJ. Improved retention of zymogen granules in cultured murine pancreatic acinar cells and induction of acinar-ductal transdifferentiation in vitro. Pancreas. 2005;30:148-157. |
| 26. | Grill J, Lamfers ML, van Beusechem VW, Dirven CM, Pherai DS, Kater M, Van der Valk P, Vogels R, Vandertop WP, Pinedo HM. The organotypic multicellular spheroid is a relevant three-dimensional model to study adenovirus replication and penetration in human tumors in vitro. Mol Ther. 2002;6:609-614. |
| 27. | Lam JT, Bauerschmitz GJ, Kanerva A, Barker SD, Straughn JM, Wang M, Barnes MN, Blackwell JL, Siegal GP, Alvarez RD. Replication of an integrin targeted conditionally replicating adenovirus on primary ovarian cancer spheroids. Cancer Gene Ther. 2003;10:377-387. |
| 28. | Zaia JA. The status of gene vectors for the treatment of diabetes. Cell Biochem Biophys. 2007;48:183-190. |