Published online Mar 14, 2009. doi: 10.3748/wjg.15.1259
Revised: October 25, 2008
Accepted: November 1, 2008
Published online: March 14, 2009
AIM: To explore whether antisense blocking of protein kinase C alpha (PKCα) would reverse multi-drug resistance (MDR) in the vincristine (VCR)-resistant human gastric cancer cell line SGC7901/VCR.
METHODS: SGC7901/VCR cells expressing antisense PKCα, SGC7901/VCR/aPKC, were established by transfection with a recombinant plasmid reversely inserted with PKCα cDNA. Empty vector (PCI-neo)-transfected cell clones, SGC7901/VCR/neo, served as the control. Western blot method was used to detect PKCα content in SGC7901, SGC7901/VCR, SGC7901/VCR/neo and SGC7901/VCR/aPKC cells, using PKCα-specific antibody. The sensitivity of SGC7901, SGC7901/VCR, SGC7901/VCR/neo and SGC7901/VCR/aPKC cells to doxorubicin (DOX) in vitro was determined by MTT assay. The uptake of DOX in these cells was detected with fluorescence spectrophotometer.
RESULTS: Western blot analysis showed that the PKCα protein level was about 8.7-fold higher in SGC7901/VCR cells than that in SGC7901 cells, whereas the protein expression of PKCα was reduced by 78% in SGC7901/VCR/aPKC cells when compared with the SGC7901/VCR cells. SGC7901/VCR/aPKC cells had a 4.2-fold increase in DOX cytotoxicity, accompanied by a 1.7-fold increase of DOX accumulation in comparison with SGC7901/VCR cells.
CONCLUSION: PKCα positively regulates MDR in SGC7901 cells, and inhibition of PKCα can partially attenuate MDR in human gastric cancer cells.
- Citation: Wu DL, Sui FY, Du C, Zhang CW, Hui B, Xu SL, Lu HZ, Song GJ. Antisense expression of PKCα improved sensitivity of SGC7901/VCR cells to doxorubicin. World J Gastroenterol 2009; 15(10): 1259-1263
- URL: https://www.wjgnet.com/1007-9327/full/v15/i10/1259.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1259
Resistance of cancer cells to chemotherapeutic drugs is a major problem in clinical treatment of cancer. Multi-drug resistance (MDR) is the most important form of drug resistance characterized by decreased cellular sensitivity to a broad range of chemotherapeutic drugs, including anthracyclines, vinca alkaloids, epipodophyllotoxins, taxanes, antibiotics and some of the new topo-I inhibitors[12]. Classical MDR is mainly caused by overexpression of P-glycoprotein (Pgp), which is coded by the MDR1 gene and functions as an ATP-dependent drug-efflux membrane transporter that rapidly extrudes a variety of hydrophobic anticancer drugs from exerting cytotoxic effect[34]. Additionally, studies have shown that MDR is also accompanied by changes in the activity of protein kinase C (PKC). Activation of PKC results in phosphorylation of Pgp and a decrease in drug accumulation, while inhibition of PKC can partially reverse the MDR phenotype[56].
PKC comprises a family of at least 12 distinct serine/ threonine kinase isoenzymes which are found in varying ratios in the cytosolic and membrane fractions of cells, depending on the type of issue and its physiological state[67]. The family of PKC is composed of three subclasses: classical, novel and atypical PKC, each having different isoforms. The members of the classical PKCs (α, βI, βII and γ) bind phorbol esters and are Ca2+ dependent. The novel PKCs (δ, ε and η) do not depend on Ca2+ but bind phorbol esters. The third subfamily includes the atypical PKCs (τ, ζ, λ and &mgr;), which do not bind to either Ca2+ or phorbol ester. It is likely that each isoform has a specific role in a given cell[57]. PKC regulates numerous cell processes including proliferation, apoptosis and differentiation by phosphorylating proteins in response to transmembrane signals from hormones, growth factors, neuro-transmitters and pharmacological agents[5]. Recently accumulated evidence indicated that PKC, especially classical PKC, plays a significant role in the formation of cancer MDR[56]. The isoenzymes of PKC possess distinct differences in localization in different cells, and research on distinct function of isoforms in cancer MDR has important significance[56].
Among those isoforms of PKC, PKCα is likely to play a decisive role in maintaining MDR phenotypes in some cancer cells, and may therefore represent potential novel targets for the treatment of cancers[68–12]. In an effort to see whether down-regulation of a single isoform of PKC could affect drug resistance, vincristine (VCR)-resistant human gastric carcinoma cell line SGC7901/VCR was transfected with an expression vector containing the cDNA for PKCα in the antisense orientation, and their expression of PKCα, doxorubicin (DOX) sensitivity and DOX accumulation were determined.
MTT and DOX were purchased from Sigma, VCR from the Twelfth Shanghai Pharmaceutical Product Factory, Lipofectamine 2000 from Invitrogen, nitrocellulose membranes and 3’, 3’-diaminobenzidene (DAB) from Sigma, G418 and rabbit polycolonal anti-PKCα from Gibeco-BRL. Plasmid pSP64-PKCα was kindly provided by Dr. PJ Parker (Imperial Cancer Foundation, England). The eukaryotic expression vector, plasmid PCI-neo, was kindly provided by Dr. Jun-Jie Xu (Institute of Microbiology and Epidemiology, Chinese Academy of Military Medical Sciences). The human gastric cancer cell line SGC7901, and its VCR-resistant counterpart SGC7901/VCR selected by stepwise exposure of parental SGC7901 cells to increasing concentrations of VCR, were purchased from Wuhan University Type Culture Collection (Wuhan, China).
Both SGC7901 and SGC7901/VCR cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere of 5% CO2. The SGC7901/VCR cells were cultured in the presence of 0.8 &mgr;mol/L VCR and grown in drug-free medium 2 wk before the experiments.
The full-length cDNA encoding PKCα cDNA (2.3 kb) was recovered from SalI sites in pSP64-PKCα plasmid and subcloned into SalI sites of PCI-neo plasmid. The recombinant plasmid with the PKCα cDNA in the antisense orientation was confirmed by BamH1 and/or SalI restriction digestion, and designated PCI-neo-aPKCα.
The process was performed as described by Wang et al[13]. Briefly, SGC7901/VCR cells were seeded in six-well plates to 70%-80% confluence. Empty vector PCI-neo and antisense vector PCI-neo-aPKCα were transfected into SGC7901/VCR cells via Lipofectamine 2000. The two kinds of transfected cells were named SGC7901/VCR /neo and SGC7901/VCR/aPKC respectively. After two days of transfection, cells were selected by culture medium containing G418 (400 mg/L) for 2 wk. The single clone was picked out using limiting dilution method and was expanded and maintained in medium containing 400 mg/L G418 until 1 wk prior to experiments.
The whole cell lysates were extracted with lysis buffer containing 1% Triton-100, 50 mmol/L NaCl, 50 mmol/L sodium fluoride, 20 mmol/L Tris (pH 7.4), 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L sodium vanadate, 0.2 mmol/L phenylmethylsulfonyl fluoride, and 0.5% NP40. An aliquot was taken for protein determination, and the remainder was mixed (1:1) with 2 × SDS sample buffer and boiled for 5 min. Samples were run on 10% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. After blocking, the membranes were incubated with rabbit polycolonal antibody against PKCα isoform at a dilution of 1:1000. Blots were washed three times with PBST to block nonspecific binding sites and incubated with secondary antiserum (goat anti-rabbit IgG conjugated to horseradish peroxidase) for 1 h at 37°C, then washed three times with 50 mmol/L Tris-HCl (pH 6.8). Color was developed with DAB as the substrate.
The cells in exponential growth were seeded in 96-well plates at a density of 2 × 104 cells per well and 24 h later graded DOX (at the concentrations of 0, 0.01, 0.03, 0.1, 0.3, 1.0, 3.0, 10 &mgr;mol/L) were added, respectively. The total medium volume of each well was 200 &mgr;L. Three days after drug addition, 10 &mgr;L MTT (5.0 g/L in PBS) was added. After 4 h of incubation, supernatants were removed and replaced by 150 &mgr;L DMSO. After formazan solubilization, the absorbance at 570 nm was recorded using an automated microplate reader. IC50 (concentration resulting in 50% inhibition of cell growth) values for DOX were calculated as 100% from plotted results using untreated cells.
The assay was performed as described by our previous report[2]. Briefly, 1.0 × 106 cells were exposed to 4.0 &mgr;mol/L DOX for 60 min. Following incubation, the cells were washed twice with ice-cold PBS, then suspended in 6.0 mL of 50% ethanol -0.3 mol/L hydrochloride, extracted for 2 h, centrifugated at 100 ×g for 10 min, and the supernatant was collected. DOX-associated mean fluorescence intensity (MFI) was measured by fluorescence spectrophotometer at an excitation wavelength of 488 nm and an emission wavelength of 590 nm. The DOX standard solution (0.1-1.0 × 103 nmol/L) was prepared, and a standard work curve with related quotient was constructed.
Data were expressed as mean ± SD. Student’s t test was used to assess statistical significance of differences. P < 0.05 was considered statistically significant.
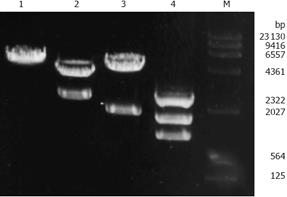
To construct the PKCα antisense expression plasmid, the full-length cDNA of PKCα (2.3 kb) recovered from SalI sites in pSP64-PKCα plasmid was subcloned into SalI sites of the eukaryotic expression vector PCI-neo (5.5 kb). A recombinant plasmid with PKCα cDNA in the antisense orientation was obtained and designated PCI-neo-aPKCα, which contains the neomycin resistance gene neo for drug selection. Two fragments were obtained by digesting PCI-neo-aPKCα with SalI, one was PKCα cDNA fragment about 2.3 kb and the other was PCI-neo cDNA fragment about 5.5 kb (Figure 1). And the inserted fragment was verified by sequencing (data not shown). SGC7901/VCR cells were transfected with either PCI-neo or PCI-neo-aPKCα, respectively, and G418-resistant clones were isolated and amplified after G418 selection. Empty vector-transfected cell clones were named SGC7901/VCR/neo, which together with the parental cell line served as the controls for these experiments. Clones transfected with the PKCα antisense expression plasmid (PCI-neo-aPKCα) were named SGC7901/VCR/aPKC.
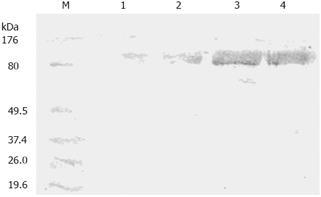
Western blot analysis was performed to assess protein abundance of PKCα in SGC7901, SGC7901/VCR, SGC7901/VCR/aPKC and SGC7901/VCR/neo cells. As shown in Figure 2, the PKCα protein level was about 8.7-fold higher in SGC7901/VCR cells than that in SGC7901 cells. Transfection of SGC7901/VCR cells with the control vector did not alter PKCα expression, whereas the protein expression of PKCα was reduced by 78% in SGC7901/VCR/aPKC cells when compared with the SGC7901/VCR cells.
Using the MTT assay, the in vitro cytotoxicity of DOX in SGC7901, SGC7901/VCR, SGC7901/VCR/neo and SGC7901/VCR/aPKC cells was examined. As shown in Table 1, SGC7901/VCR cells were 23.5 times more resistant to DOX in comparison with SGC7901 cells. The IC50 in SGC7901/VCR/aPKC was 4.2-fold lower than that in SGC7901/VCR cells, indicating that antisense PkCα could partially reverse resistance to DOX in multi-drug resistant SGC7901 cells.
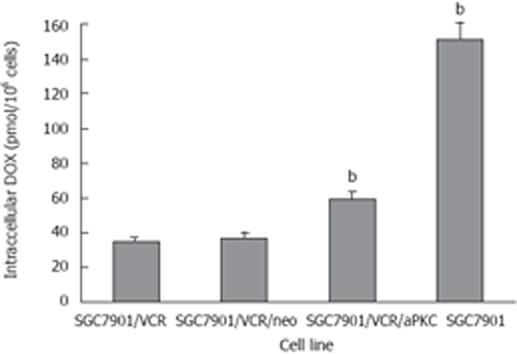
DOX accumulation in SGC7901, SGC7901/VCR, SGC7901/VCR/neo cells and SGC7901/VCR/aPKC was measured by fluorescence spectrophotometry after incubation of cells with 4.0 &mgr;mol/L DOX for 60 min. As shown in Figure 3, SGC7901/VCR cells accumulated 4.3-fold less DOX than SGC7901 cells, whereas SGC7901/VCR/aPKC cells showed 1.7-fold more drug retention than SGC7901/VCR cells.
MDR to cancer treatment has been studied for more than 20 years. No useful method of reversing MDR suitable for clinical use has yet emerged from this large quantity of work. The reason could be complicated mechanisms involved. There are several ways for cancer cells to develop MDR[4]. The most investigated mechanisms with known clinical significance are: (1) activation of transmembrane proteins effluxing different chemical substances from the cells, in which Pgp is the best known efflux pump; (2) activation of the enzymes of the glutathione detoxification system; (3) alterations of the genes and the proteins involved in the control of apoptosis (especially p53 and Bcl-2)[10]. Recent studies have indicated a role for PKC in the regulation of the MDR phenotype. A number of studies have demonstrated that PKC activity was elevated in MDR-selected cell lines, and several PKC inhibitors are able to partially reverse MDR and inhibit Pgp phosphorylation[56]. Pgp is phosphorylated by PKC and that phosphorylation positively modulates its transport function[14]. The PKC family consists of at least 12 isoforms, and different isoforms of PKC possess distinct differences in expression and function in different MDR cells[12–15]. The elevated PKC activity level of the MDR cancer cells was frequently a result of increased PKCα expression in most cancers[8–12], but in some cancers it was due to enhanced expression of other isoforms, such as PKCβI, PKCγ or PKCη[15–18].
SGC7901/VCR is an established VCR-resistant cell line selected by stepwise exposure of parental SGC7901 cells to increasing concentrations of VCR. Previous reports have proved that the SGC7901/VCR cells possess the characteristics of classical MDR with overexpression of Pgp, and the SGC7901/VCR cell line has been successfully used as an in vitro MDR reversal model by several study groups, including ours[19–23]. The recent report showed that the expression of PKCα was significantly higher in SGC7901/VCR cells than in SGC7901 cells, and there was no significant difference in the expression of PKCβI, PKCβII and PKCγ between SGC7901/VCR and SGC7901 cells[10].
In the present study, we observed that the expression of PKCα was significantly higher in SGC7901/VCR cells than in SGC7901 cells, which was consistent with the previous report by Han et al[10]. To investigate the possible effect of PKCα on MDR for chemotherapeutic drug in human gastric cancer cells, we established SGC7901/VCR cells stably expressing antisense PKCα. We demonstrated that down-regulation of the predominant PKCα isoform expressed in SGC7901/VCR cells by the expression of antisense PKCα led to greater DOX accumulation and partial reversal of resistance to DOX. These data support the thesis that MDR in this cell line is modulated, in part, by PKCα. These results suggest that a more effective means of reducing PKCα activity, possibly with RNA interference, might be a more efficient way of increasing drug sensitivity in this MDR cell line.
Protein kinase C (PKC) constitutes a family of closely related protein serine/threonine kinase which are sub-grouped into classical (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ι and ζ) isoforms. The members of the PKC family are involved in the regulation of numerous cell processes including proliferation, apoptosis, and differentiation. It is likely that each isoform has a specific role in a given cell.
A recent study confirmed that PKCα, PKCβI, PKCβII and PKCγ were expressed in both human gastric carcinoma cell line SGC7901 and vincristine (VCR)-resistant human gastric carcinoma cell line SGC7901/VCR, but the expression of PKCα was significantly higher in SGC7901/VCR cells than that in SGC7901 cells. There was no significant difference in the expression of PKC-βI, PKC-βII and PKCγ between SGC7901/VCR cells and SGC7901 cells. In this report, the authors transfected SGC7901/VCR cells with an expression vector containing the cDNA for PKCα in the antisense orientation to see whether down-regulation of PKCα could affect drug resistance in SGC7901/VCR cells.
In the present study the authors observed that down-regulation of the predominant PKCα isoform expressed in SGC7901/VCR cells by the expression of antisense PKCα led to greater doxorubicin accumulation and partial reversal of resistance to doxorubicin. These data support the thesis that MDR in this cell line is modulated in part by PKCα.
This study provided a new target against MDR in human gastric carcinoma, suggesting that a more effective means of reducing PKCα activity, possibly with RNA interference, might be a more efficient way of increasing drug sensitivity in this MDR cell line.
The manuscript entitled “Antisense expression of PKCα improved sensitivity of SGC7901/VCR cells to doxorubicin” by Wu et al examines the effects of antisense inhibition of PKCα expression on the MDR phenotype of gastric cancer cells. The authors used antisense technology to establish a derivative of the multi-drug/vincristine-resistant SGC7901-VCR cell line with reduced expression of PKCα. Using this isogenic pair of cell lines, the authors conclude that PKCα deficiency leads to increased intracellular accumulation of DOX and partially restores sensititivity to the drug, thus reversing the MDR phenotype.
| 1. | Wu DL, Huang F, Lu HZ. [Drug-resistant proteins in breast cancer: recent progress in multidrug resistance]. Ai Zheng. 2003;22:441-444. |
| 2. | Wu DL, Li MJ, Gao HZ, Wu DZ. Daunorubicin-albumin conjugate reverses resistance in multidrug resistant KB/VCR cells to daunorubicin. Zhongguo Yaoli Duli Zazhi. 1997;11:54-58. |
| 3. | Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105-127. |
| 4. | Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273-286. |
| 5. | O’Brian CA, Ward NE, Stewart JR, Chu F. Prospects for targeting protein kinase C isozymes in the therapy of drug-resistant cancer--an evolving story. Cancer Metastasis Rev. 2001;20:95-100. |
| 6. | Fine RL, Chambers TC, Sachs CW. P-glycoprotein, multidrug resistance and protein kinase C. Stem Cells. 1996;14:47-55. |
| 7. | Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006;235:1-10. |
| 8. | Blobe GC, Sachs CW, Khan WA, Fabbro D, Stabel S, Wetsel WC, Obeid LM, Fine RL, Hannun YA. Selective regulation of expression of protein kinase C (PKC) isoenzymes in multidrug-resistant MCF-7 cells. Functional significance of enhanced expression of PKC alpha. J Biol Chem. 1993;268:658-664. |
| 9. | Cloud-Heflin BA, McMasters RA, Osborn MT, Chambers TC. Expression, subcellular distribution and response to phorbol esters of protein kinase C (PKC) isozymes in drug-sensitive and multidrug-resistant KB cells evidence for altered regulation of PKC-alpha. Eur J Biochem. 1996;239:796-804. |
| 10. | Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441-445. |
| 11. | Lahn M, Kohler G, Sundell K, Su C, Li S, Paterson BM, Bumol TF. Protein kinase C alpha expression in breast and ovarian cancer. Oncology. 2004;67:1-10. |
| 12. | Lahn M, Sundell K, Kohler G. The role of protein kinase C-alpha in hematologic malignancies. Acta Haematol. 2006;115:1-8. |
| 13. | Wang XY, Repasky E, Liu HT. Antisense inhibition of protein kinase Calpha reverses the transformed phenotype in human lung carcinoma cells. Exp Cell Res. 1999;250:253-263. |
| 14. | Yang JM, Chin KV, Hait WN. Interaction of P-glycoprotein with protein kinase C in human multidrug resistant carcinoma cells. Cancer Res. 1996;56:3490-3494. |
| 15. | Aquino A, Warren BS, Omichinski J, Hartman KD, Glazer RI. Protein kinase C-gamma is present in adriamycin resistant HL-60 leukemia cells. Biochem Biophys Res Commun. 1990;166:723-728. |
| 16. | Gollapudi S, Soni V, Thadepalli H, Gupta S. Role of protein kinase beta isozyme in multidrug resistance in murine leukemia P388/ADR cells. J Chemother. 1995;7:157-159. |
| 17. | Svensson K, Larsson C. A protein kinase Cbeta inhibitor attenuates multidrug resistance of neuroblastoma cells. BMC Cancer. 2003;3:10. |
| 18. | Sonnemann J, Gekeler V, Ahlbrecht K, Brischwein K, Liu C, Bader P, Muller C, Niethammer D, Beck JF. Down-regulation of protein kinase Ceta by antisense oligonucleotides sensitises A549 lung cancer cells to vincristine and paclitaxel. Cancer Lett. 2004;209:177-185. |
| 19. | Yang YX, Xiao ZQ, Chen ZC, Zhang GY, Yi H, Zhang PF, Li JL, Zhu G. Proteome analysis of multidrug resistance in vincristine-resistant human gastric cancer cell line SGC7901/VCR. Proteomics. 2006;6:2009-2021. |
| 20. | Zhao Y, Xiao B, Chen B, Qiao T, Fan D. Upregulation of drug sensitivity of multidrug-resistant SGC7901/VCR human gastric cancer cells by bax gene transduction. Chin Med J (Engl). 2000;113:977-980. |
| 21. | Gao FL, Wang F, Wu JL, LE XP, Zhang QX. [Screening effective sequences of small interfering RNAs targeting MDR1 gene in human gastric cancer SGC7901/VCR cells]. Zhonghua Zhongliu Zazhi. 2006;28:178-182. |
| 22. | Tang XQ, Bi H, Feng JQ, Cao JG. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 2005;26:1009-1016. |
| 23. | Wu DL, Xu Y, Yin LX, Lu HZ. Reversal of multidrug resistance in vincristine-resistant human gastric cancer cell line SGC7901/VCR by LY980503. World J Gastroenterol. 2007;13:2234-2237. |