Published online Mar 14, 2009. doi: 10.3748/wjg.15.1254
Revised: January 7, 2009
Accepted: January 14, 2009
Published online: March 14, 2009
AIM: To review the literature on capsule endoscopy (CE) for detecting esophageal varices using conventional esophagogastroduodenoscopy (EGD) as the standard.
METHODS: A strict literature search of studies comparing the yield of CE and EGD in patients diagnosed or suspected as having esophageal varices was conducted by both computer search and manual search. Data were extracted to estimate the pooled diagnostic sensitivity and specificity.
RESULTS: There were seven studies appropriate for meta-analysis in our study, involving 446 patients. The pooled sensitivity and specificity of CE for detecting esophageal varices were 85.8% and 80.5%, respectively. In subgroup analysis, the pooled sensitivity and specificity were 82.7% and 54.8% in screened patients, and 87.3% and 84.7% in the screened/patients under surveillance, respectively.
CONCLUSION: CE appears to have acceptable sensitivity and specificity in detecting esophageal varices. However, data are insufficient to determine the accurate diagnostic value of CE in the screen/surveillance of patients alone.
- Citation: Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol 2009; 15(10): 1254-1258
- URL: https://www.wjgnet.com/1007-9327/full/v15/i10/1254.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1254
Variceal bleeding is a significant contributor to the morbidity and mortality associated with cirrhosis and portal hypertension. It has been estimated that up to 90% of patients with cirrhosis will ultimately develop esophageal varices[1]. Once the varices develop, the occurrence of subsequent variceal bleeding in the next 24 mo is 25%-35%, and the risk of the patient dying as a result of the index hemorrhage is up to 50%[23]. Esophagogastroduodenoscopy (EGD) is the most effective method of evaluating patients with portal hypertension and cirrhosis[4]. However, the procedure is unpleasant, and still associated with a small but potential risk of complications[5]. In addition, it is often carried out with the patients under sedation, which may bring additional complications in patients with cirrhosis[6].
The PillCam ESO (Given Imaging, Israel) (esophageal capsule endoscopy, ECE) is a novel, wireless endoscope, similar in size to that of the standard PillCam small-bowel capsule endoscope (SBCE), which acquires video-images from both ends of the device during passage through the esophagus, and it has been approved by the United States Food and Drug Administration[7]. ECE provides a potentially less invasive diagnostic alternative in evaluating diseases of the esophagus such as esophageal varices, Barrett’s esophagus, and gastroesophageal reflux disease (GERD)/erosive esophagitis[8–11]. Furthermore, Ramirez et al[12] attached a string to SBCE in the middle of the device which can allow the capsule to move up and down in the esophagus by swallowing the capsule and pulling the string. This improvement can extend the retention time of CE in the esophagus and provide adequate information about esophageal details.
Recent years, a number of studies have been performed for evaluating CE in diagnosing esophageal disease, especially for Barrett’s esophagus and esophageal varices, and the results varied[8–10]. If CE is a definitive diagnostic tool for esophageal varices, it is necessary to evaluate whether CE is sufficiently accurate for this purpose. In order to estimate the sensitivity and specificity of CE in the diagnosis of esophageal varices, a systematic review and meta-analysis of studies using CE for detecting esophageal varices with EGD as the standard was conducted.
A search in MEDLINE, EMBASE and ENBASE reviews (CDSR, ACP Journal Club, DARE, CCTR, CLCMR, CLHTA and CLEED) was conducted in May 2008. We did not confine our search to English language publications. Thorough literature retrieval for studies comparing CE and EGD for detecting esophageal varices in patients diagnosed or suspected as having esophageal varices was conducted along with a search of reference lists.
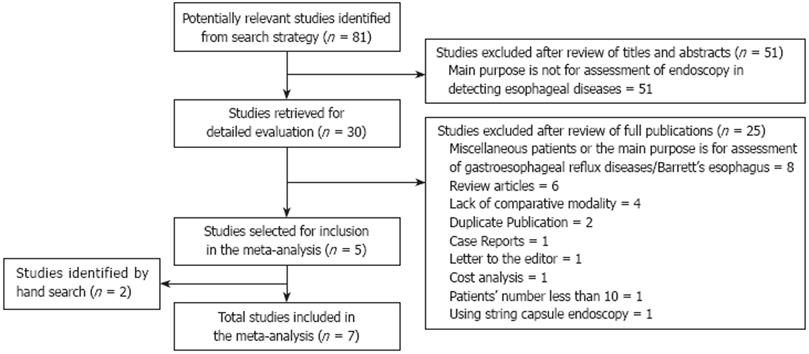
An additional search of abstracts presented at the proceedings of Digestive Disease Week (DDW) from 2004 to 2008 and international conference on CE through 2004 to 2007 was performed. If multiple updates of the same data were found, we selected only the most recent version or the most complete data for analysis. The search strategy employed is shown in Figure 1.
Predefined criteria had to be met for the studies to be included. Studies comparing CE diagnostic accuracy in patients diagnosed or suspected as having esophageal varices with EGD as standard were included. CE frames were assessed by an investigator who was blinded to patient’s EGD findings. The studies which met the following one or more criteria were excluded: (1) esophageal varices were detected by CE but were performed in obscure gastrointestinal bleeding patients or for assessment of small bowel diseases or in miscellaneous patients with esophageal diseases; (2) string CE was used to detect esophageal varices; and (3) studies with patient number less than 10.
The study parameters were extracted first independently and subsequently in consensus if there was a disagreement between the reviewers (Liao Z, Gao R) concerning the numeric value of a parameter. Data were extracted based on the previous reported standards. The absolute number of true-positive, false-positive, true-negative and false-negative was retrieved or calculated with Bayes theorem if values for sensitivity and specificity and predictive values were reported. These were also performed separately by the two reviewers and subsequently checked for an agreement. The full text of papers (if available) of all relevant studies were obtained.
Meta-analysis was undertaken using MetaDiSc statistical software (Meta-DiSc, version 1.4, Madrid, Spain) to estimate the overall sensitivity and specificity. χ2 test was then performed to test for heterogeneity between studies, P value less than 0.1 was considered significant for heterogeneity. Wherever zero counts occurred for study data, a continuity correction of 0.5 was added to each value for that study. In order to define the calculation of sensitivity and specificity, fixed effect model was used when P value was more than 0.1 for heterogeneity test and random effect model used for P value was less than 0.1[13].
Eighty-one articles were selected through original searches in MEDLINE, EMBASE and ENBASE reviews (CDSR, ACP Journal Club, DARE, CCTR, CLCMR, CLHTA and CLEED) databases. Fifty-one articles were excluded after review of the titles and abstracts, leaving 30 articles for detailed evaluation by two independent reviewers. Of these, five met our inclusion criteria[1014–17]. Two studies were identified by hand search in DDW 2008 meeting abstracts and reference lists respectively[1819]. In total, seven studies, involving 446 patients were appropriate for meta-analysis. In the seven studies, CE type was PillCam ESO without string and they were all published in English. One study was performed in western Europe[14], one in Australia[15], two were international multi-center studies[1017] and three in the USA[161819]. The patient inclusion/exclusion criteria were all reported in these studies. All patients were for clinically indicated screening (suspected) or surveillance (diagnosed) of esophageal varices. EGD was performed after CE in the same day for most patients, and all patients in these studies served as their own controls. All endoscopists who assessed the CE images were blinded to the EGD diagnoses. CE transit time was variable from 134.5 s (median) to 251 s (Table 1).
| Author and year of publication | Patients (screening) | Country | Article type | Study design | Setting | Patients inclusion/exclusion criteria reported | Two methods performed time | Transit time in esophagus |
| Lapalus, 2006 | 20 (20) | France | O | P, B | S | Yes | EGD after ECE on the same day | 213 s |
| Eisen, 2006 | 32 (10) | International | O | P, B | 3 centers | Yes | EGD after ECE on the same day | 134.5 s (median) |
| Smith, 2007 | 15 (15) | Australia | A | NR, B | S | Yes | EGD within 1-2 h after ECE | NR |
| Groce, 2007 | 21 (21) | USA | A | P, B | S | Yes | EGD immediately after ECE | 251 s |
| Pena, 2008 | 20 (8) | USA | O | P, B | S | Yes | EGD within 1h after ECE | NR |
| Jensen, 2008 | 50 (50) | USA | O | P, B | S | Yes | NR | NR |
| de Franchis, 2008 | 288 (195) | International | O | P, B | 11 centers | Yes | EGD within 48 h after ECE | NR |
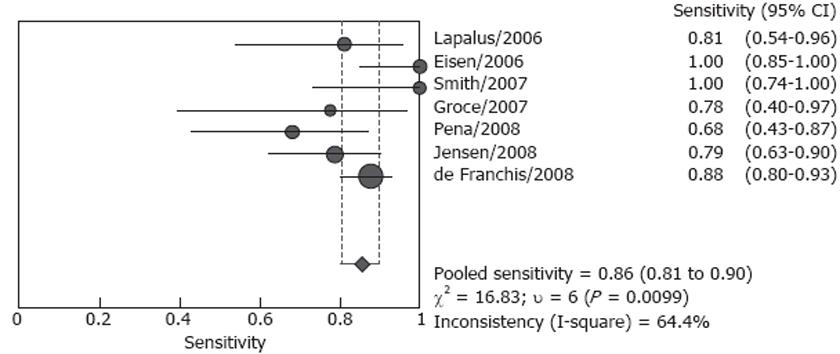
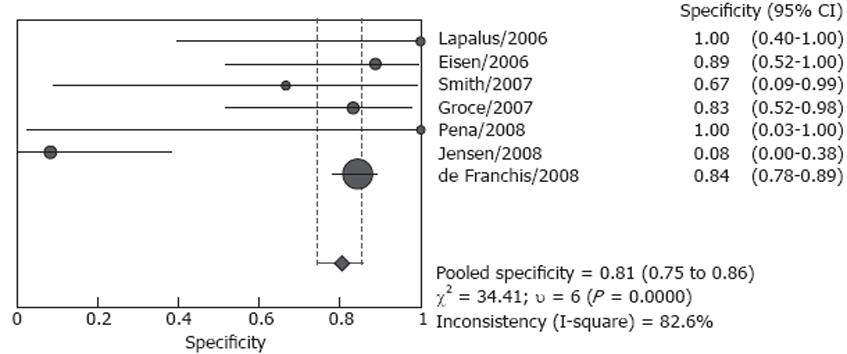
Forrest plots for sensitivity and specificity are shown in Figures 2 and 3. Reported sensitivity ranged from 68.4% to 100% in the individual studies, while specificity ranged from 8.3% to 100%. The pooled estimate of sensitivity was 85.8% (95% CI: 80.5%-90.1%) and the pooled estimate of specificity was 80.5% (95% CI: 74.7%-85.5%). However, both estimates were subject to significant heterogeneity (P = 0.010 and P < 0.001, respectively). In the presence of such heterogeneity, pooled estimates should be interpreted with caution.
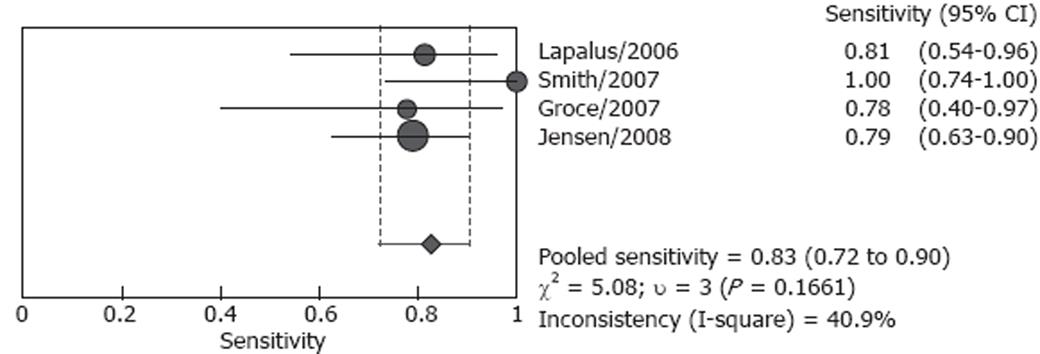
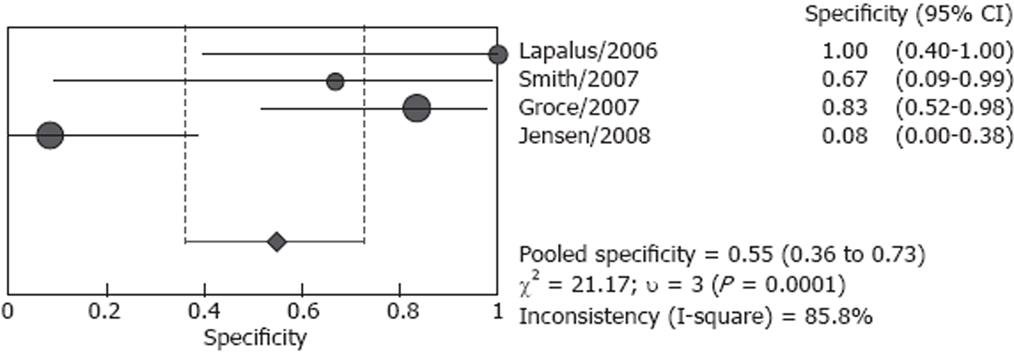
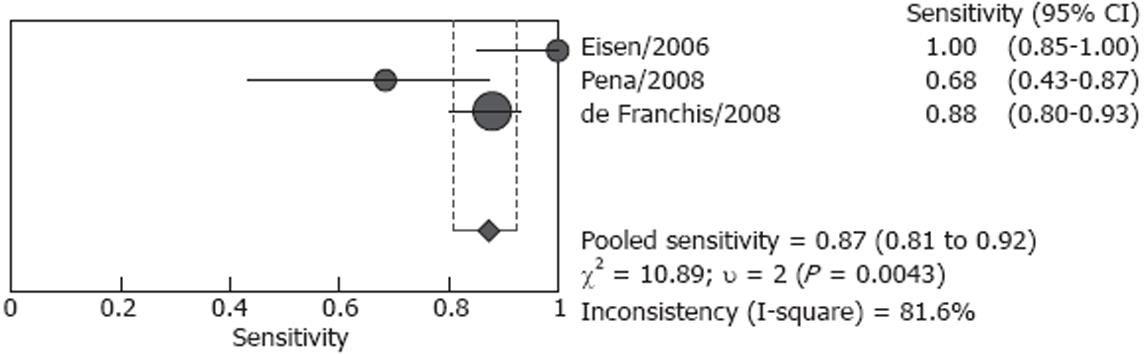
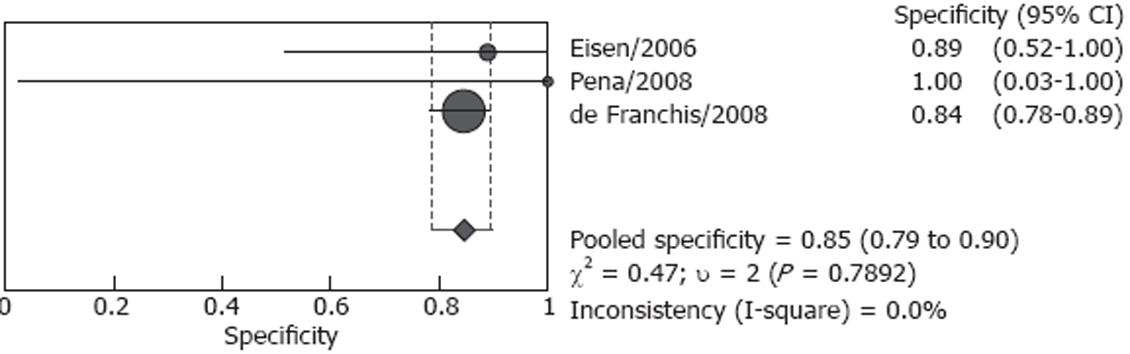
Studies included in our analysis were further classified into two subgroups: screening group and screening/surveillance group. There were four studies including 106 patients in the screening group, no heterogeneity (P = 0.166) was found for the analysis of sensitivity, and the pooled sensitivity was 82.7% (95% CI: 72.2%-90.4%), however, heterogeneity was found significant for specificity (P < 0.001), the pooled result was 54.8 % (95% CI: 36.0%-72.7%) (Figures 4 and 5). Both screened patients and patients under surveillance were included in the other three studies, involving 340 patients, and the detailed endoscopic results cannot be independently extracted for screen patients or surveillance patients. The pooled sensitivity was 87.3% (95% CI: 80.9%-92.2%) in a random effect model (P = 0.004) and pooled specificity was 84.7% (95% CI: 78.8%-89.5%) in a fixed effect model (P = 0.789). (Figures 6 and 7)
Meta-analysis has been performed for SBCE in detecting small bowel disease comparing with other methods. It is reported SBCE was superior when compared with push enteroscopy and small bowel barium radiography for OGIB (P < 0.00001)[20]. In detecting Crohn’s disease, SBCE was also superior to small bowel barium radiography (P < 0.001), colonoscopy with ileoscopy (P = 0.02) and CT (P = 0.001)[21]. Furthermore, the yield of SBCE for small bowel diseases was similar to double-balloon enteroscopy in combination with oral and anal approaches[22].
In the largest study performed in 11 centers (288 patients), the sensitivity and specificity were 88.0% and 84.4%, respectively, and ECE had good agreement with EGD[17]. The present meta-analysis indicates that CE appears to have acceptable sensitivity and specificity for esophageal varices with EGD as the standard. However, the limitations of these data need to be appreciated. Firstly, because this new device has not been widely used for detecting esophageal varices, and the number of studies was small. Secondly, sample sizes of different studies were significantly different, ranging from 15 to 288 patients. So we considered the P value less than 0.1 as significant for heterogeneity in this analysis. At last, patients underwent CE including both screened patients and patients under surveillance, in 3 studies the detailed endoscopic results can not be extracted for these patients, and the accurate pooled diagnostic value of CE for patients under surveillance was not known.
There was considerable heterogeneity between the studies. In Pena’s and Groce’s studies, the sensitivity of ECE in detecting esophageal varices was only 68.4% and 77.8% respectively[1618]. In Eisen’s and Smith’s studies, the sensitivity was both as high as 100%[1015]. The diagnostic specificity of these seven studies varied significantly, the specificity was lowest in Jensen’s study, only 8.3%, and the accuracy of capsule for detecting esophageal varices was modest[19]. However, in Lapalus’s[14] and Pena’s[16] studies, the specificity was both 100%. There was no heterogeneity in the analysis of sensitivity and specificity for the screening group and the screening/surveillance group, respectively. Although the sample was too small to undertake meta-regression to identify the cause of the heterogeneity, some potential reasons for heterogeneity may be identified. Except for the small sample size of most studies, the most possible causes for the existence of heterogeneity may be related to the experience of the endoscopists and the number of endoscopists who read the capsule images. Although the ECE investigators were all blinded to EGD findings, their experience was not described in detail. Only in Pena’s study, the endoscopists were those with more than 5 years of experience in reading SBCE but no experience in reading ECE[16]. In two studies, ECE images were reviewed by two independent investigators[1419], and one investigator in an other five studies[1019].
The pooled sensitivity and specificity of the screening/surveillance group was higher than the screening group. The pooled data of screened patients may be confused by Jensen’s study, which had the largest number of patients in the screening studies and with a specificity of 8.3%[19]. In studies with screening/surveillance patients, the detailed results cannot be extracted, so the pooled data of surveillance patients cannot be obtained. One defect in the three studies was the percentages of screening or surveillance patients that were variable (Figure 1).
In summary, CE appears to have acceptable sensitivity and specificity in detecting esophageal varices, however, it seems inaccurate in screening patients based on the present data. There was insufficient data to determine the accurate diagnostic value of CE in patients under surveillance alone. Also, further researches with large numbers of patients are needed.
Capsule endoscopy (CE) has been reported to play an important role in detecting esophageal diseases, especially for esophageal varices. There are concerns about whether CE is sufficiently accurate for evaluating this desease.
Data concerning CE in detecting esophageal varices with esophagogastroduodenoscopy (EGD) results as the standard were derived from published original papers or conference abstracts. A meta-analysis was conducted to estimate the sensitivity and specificity of CE in diagnosis of esophageal varices.
The current study demonstrated that CE appears to have acceptable sensitivity and specificity in detecting esophageal varices. However, it seems inaccurate in screening patients.
The diagnostic accuracy of CE was low in screening patients in this study. Studies for further evaluating CE diagnostic accuracy in screening patients alone were needed.
Esophageal capsule endoscope (ECE) is a novel, wireless endoscope, similar in size to small-bowel capsule endoscope, which acquires video-images from both ends of the device during passage through the esophagus.
This meta-analysis was performed based on five peer reviewed papers and 2 in abstract form, involving about 500 patients. This study was well done, however, the missing rate of the small-sized varices is high and this tool it suitable for medium- and large-sized varices.
| 1. | Rigo GP, Merighi A, Chahin NJ, Mastronardi M, Codeluppi PL, Ferrari A, Armocida C, Zanasi G, Cristani A, Cioni G. A prospective study of the ability of three endoscopic classifications to predict hemorrhage from esophageal varices. Gastrointest Endosc. 1992;38:425-429. |
| 2. | Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. N Engl J Med. 1988;319:983-989. |
| 3. | Gores GJ, Wiesner RH, Dickson ER, Zinsmeister AR, Jorgensen RA, Langworthy A. Prospective evaluation of esophageal varices in primary biliary cirrhosis: development, natural history, and influence on survival. Gastroenterology. 1989;96:1552-1559. |
| 4. | de Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis. 2004;36:787-798. |
| 5. | Lapalus MG, Saurin JC. [Complications of gastrointestinal endoscopy: gastroscopy and colonoscopy]. Gastroenterol Clin Biol. 2003;27:909-921. |
| 6. | Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut. 1991;32:12-15. |
| 7. | Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545. |
| 8. | Eliakim R, Yassin K, Shlomi I, Suissa A, Eisen GM. A novel diagnostic tool for detecting oesophageal pathology: the PillCam oesophageal video capsule. Aliment Pharmacol Ther. 2004;20:1083-1089. |
| 9. | Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, Sachdev R, Mitty RD, Hartmann D, Schilling D. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572-578. |
| 10. | Eisen GM, Eliakim R, Zaman A, Schwartz J, Faigel D, Rondonotti E, Villa F, Weizman E, Yassin K, deFranchis R. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy. 2006;38:31-35. |
| 11. | Mata A, Llach J, Bordas JM. Wireless capsule endoscopy. World J Gastroenterol. 2008;14:1969-1971. |
| 12. | Ramirez FC, Hakim S, Tharalson EM, Shaukat MS, Akins R. Feasibility and safety of string wireless capsule endoscopy in the diagnosis of esophageal varices. Am J Gastroenterol. 2005;100:1065-1071. |
| 13. | Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. |
| 14. | Lapalus MG, Dumortier J, Fumex F, Roman S, Lot M, Prost B, Mion F, Ponchon T. Esophageal capsule endoscopy versus esophagogastroduodenoscopy for evaluating portal hypertension: a prospective comparative study of performance and tolerance. Endoscopy. 2006;38:36-41. |
| 15. | Smith BW, Jeffrey GP, Adams LA, Leber J, Blanchard J, Garas G. Utilisation of capsule endoscopy in variceal screening and surveillance. J Gastroenterol Hepatol. 2007;22:A343. |
| 16. | Pena LR, Cox T, Koch AG, Bosch A. Study comparing oesophageal capsule endoscopy versus EGD in the detection of varices. Dig Liver Dis. 2008;40:216-223. |
| 17. | de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, Vargas HE, Vargo J, Thompson J. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595-1603. |
| 18. | Groce JR, Raju GS, Sood GK, Snyder N. A prospective single blinded comparative trial of capsule esophagoscopy vs. traditional EGD for variceal screening. Gastroenterology. 2007;132:A802. |
| 19. | Jensen DM, Singh B, Chavalitdhamrong D, Kovacs TO, Carrico MM, Han SH, Durazo FA, Saab S. Is Capsule Endoscopy Accurate Enough to Screen Cirrhotics for High Risk Varices & Other Lesions? A Blinded Comparison of EGD & PillCam ESO. Gastrointest Endosc. 2008;67:AB122. |
| 20. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. |
| 21. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. |
| 22. | Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372-4378. |