Published online Mar 14, 2009. doi: 10.3748/wjg.15.1242
Revised: February 9, 2009
Accepted: February 16, 2009
Published online: March 14, 2009
AIM: To evaluate the prognostic factors for 5-year survival after local excision of rectal cancer, and to examine the therapeutic efficacy and surgical indications for this procedure.
METHODS: Clinical data, obtained from 106 local rectal cancer excisions performed between January 1980 and December 2005, were retrospectively analyzed. Survival analysis was performed using the Kaplan-Meier method, statistical comparisons were performed using the log-rank test, and multivariate analysis was performed using the Cox proportional hazards model.
RESULTS: Transanal, transsacral, and transvaginal excisions were performed in 92, 12, and 2 cases, respectively. The rate of complication, local recurrence, and 5-year survival was 6.6%, 17.0%, and 86.7%, respectively. Univariate analysis showed that T stage, vascular invasion, and local recurrence were related to the prognosis of the cases (P < 0.05). Multivariate analysis showed that T stage [P = 0.011, 95% confidence interval (CI) = 1.194-3.878] and local recurrence (P = 0.022, 95% CI = 1.194-10.160) were the major prognostic factors for 5-year survival of cases after local excision of rectal cancer.
CONCLUSION: Local rectal cancer excision is associated with few complications, and suitable for stages Tis and T1 rectal cancer. Prevention of local recurrence, active postoperative follow-up, and administration of salvage therapy are the effective methods to increase the efficacy of local excision of rectal cancer.
- Citation: Zhao DB, Wu YK, Shao YF, Wang CF, Cai JQ. Prognostic factors for 5-year survival after local excision of rectal cancer. World J Gastroenterol 2009; 15(10): 1242-1245
- URL: https://www.wjgnet.com/1007-9327/full/v15/i10/1242.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1242
In China, unlike in Western countries, rectal cancer accounts for approximately 70% of colorectal cancers. Both abdominoperineal resection and anterior resection of rectal cancer can result in postoperative urinary impairment and sexual dysfunction, and the presence of a permanent colostomy significantly impacts the quality of life. With the advancement of imaging techniques, the accuracy of preoperative rectal cancer staging has increased dramatically, and the preservation of physical function in rectal cancer patients has become a very important aim of research. Local tumor excision preserves anal, urinary, and sexual function in patients with low rectal cancer. Some patients with early tumor have suitable indications for local tumor excision[1–5]. This study was to determine the prognostic factors for survival after local excision of rectal cancer.
This study included 106 low rectal cancer patients at the age of 26-81 years (60 males, 46 females, with a median age of 60 years) who underwent local tumor excision at our hospital between January 1980 and December 2005. The main symptom on presentation was rectal bleeding (95 patients). Other symptoms included passage of mucus, altered bowel habits. Tumors were located at the anterior wall in 45 patients, posterior wall in 35 patients, and lateral wall in 21 patients, none located in 5 patients. The diameter of the tumor was ≤ 3.0 cm in 70.5% (80/106) patients and > 3.0 cm in 17.9% (19/106) patients. Colonoscopy or barium enema was performed to exclude multiple primary tumors, while ultrasound, chest X-ray, or computed tomography (CT) was conducted to exclude distant metastases, thus a pathological diagnosis was made before operation.
Transanal excision (TAE), transsacral excision (TSE), and transvaginal excision (TVE) were performed in 92, 12, and 2 patients, respectively. The margin was excised 1-2 cm from the tumor, and postoperative pathology reports indicated negative margins in all cases.
Four patients received preoperative radiotherapy and 41 patients underwent postoperative radiotherapy at the dose of 14-75 Gy (mean 53.5 Gy).
Of the 106 patients, 104 received follow-up (by outpatient appointments, telephone, or mail), and the follow-up rate was 98.1%. Follow-up was performed between 11 mo and 20 years after operation, and the median follow-up time was 72 mo. During the follow-up period, 14 patients died. Of them, 9 died of tumor metastasis, 4 of other conditions, and 1 of a second primary esophageal cancer, respectively.
SPSS 13.0 software was used for statistical processing. Survival analysis was performed using the Kaplan-Meier method, univariate analysis of prognostic factors was performed using the log-rank test, and multivariate analysis was performed using the Cox proportional hazards model. P < 0.05 was considered statistically significant.
The complication rate was 6.6% (7/106). Postoperative bleeding occurred in 1 case and wound breakdown was observed in 1 case after TSE. Four cases had anastomotic leakage after TAE, 1 of them was accompanied with bleeding. One case had rectovaginal fistulation after TVE. No death occurred during surgery.
Of the 106 patients, 18 (17%) had local recurrence 4-174 mo (mean 48.3 mo) after operation. The local recurrence rate for Tis-, T1-, and T2-stage tumors was 7.1%, 18.7%, and 20.8%, respectively (P = 0.331), while the local recurrence rate for tumors with or without vascular invasion was 66.7% and 15.5%, respectively (P = 0.02). Surgical treatment was reattempted for 12 patients after recurrence. Local excision was performed in 5 patients. Of them, 1 died after 93 mo and the other 4 survived. Abdominoperineal resection was performed in 6 patients, and Park’s procedure was performed in 1 patient. Of these 7 patients, 2 died after 36 and 40 mo, respectively, the other 5 survived. Distal metastases were present in 9 patients, who were then treated with chemotherapy, radiotherapy, or interventional therapy. Four T3 stage patients received preoperative radiotherapy, and local excision was performed after down staging. Tumors recurred in 2 patients with a recurrence rate of 50%.
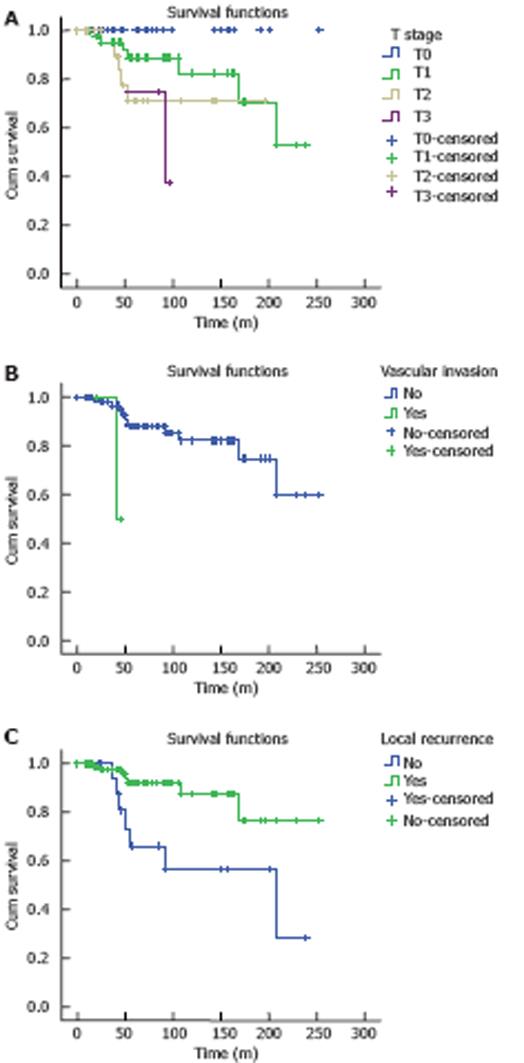
The overall 5-year survival rate for our patients was 86.7%. The 5-year survival rate for Tis-, T1-, and T2-stage tumors was 100%, 88.8%, and 67.9%, respectively. Univariate analysis showed that infiltration depth, vascular invasion, and local recurrence were the prognostic factors for tumors (P < 0.05, Figure 1). Multivariate analysis showed that T stage [P = 0.011, OR = 2.152, 95% confidence interval (CI) = 1.194-3.878] and local recurrence (P = 0.022, OR = 3.483, 95% CI = 1.194-10.160) were the major prognostic factors for tumors.
Although radical resection is an effective treatment for rectal cancer, it can lead to anal, urinary, and reproductive function impairment as well as surgical complications and death. Morson et al[6] have reported a 5-year survival rate of 82% in patients after local excision of early rectal cancer. Subsequent studies demonstrated that local excision of early stage low rectal cancer can produce a good outcome and preserve anal, urinary, and reproductive function[37–10], but a higher local recurrence rate of tumor (5%-27%) has limited its wide application. Nevertheless, the 5-year survival rate after local tumor excision reported in the literature is 72%-95%[37–10]. In our study, the local recurrence rate was 17.0% and the 5-year survival rate was 86.7%, respectively, which are similar to the reported data[37–10].
Risk factors affecting low rectal cancer prognosis after excision include the age and sex of patients, treatment methods, and vascular invasion and tumor stage. In our study, the age and sex of patients were unrelated to the prognosis of low rectal cancer. Vascular invasion and tumor stage affected rectal cancer recurrence and prognosis after local excision. Bouvet et al[11] found that T stage is the most important pathological factor for local rectal cancer recurrence after excision. In our patients, excision of Tis, T1, and T2 tumors was associated with the 5-year survival rate (P < 0.05). The local recurrence and distant metastasis rates were significantly increased when the tumor was poorly differentiated, macroscopically ulcerative, or larger than 3.0 cm in diameter. However, because the number of patients was small in our study, the differences were not statistically significant. It was reported that rectal cancer patients also have a higher rate of lymphatic spread[1213], and therefore local excision should be recommended with caution for such patients.
The excision method of local tumor may affect its prognosis. If R0 tumor excision is achieved, the surgical method used does not affect its local recurrence rate or the survival rate of patients. In our patients, all margins on pathological examination were negative. It was reported that if tumor margin is positive, the recurrence rate of tumor will be high and its prognosis is poor[14]. Therefore, if the margin is positive or borderline positive, the excision margins should be widened, otherwise, local tumor excision should be abandoned. Frozen section examination of margins during surgery ensures margin negativity, which is essential for complete local tumor excision. It was reported that adjuvant chemotherapy and radiotherapy for tumor after local excision can reduce its local recurrence rate[15]. Our data show that radiotherapy was unrelated to local recurrence and prognosis of tumor, which may be due to the different indications for radiotherapy, and the standardized treatment modalities. It is generally believed that rectal cancer patients should undergo adjuvant chemotherapy and radiotherapy after local excision of T2 rectal tumor and poorly-differentiated or high-risk T1 rectal tumor[16].
Salvage therapy is of great importance in the treatment of recurrent rectal tumor after local excision and can be performed when rectal wall involvement is significantly greater than extramural or pelvic cavity involvement. Therefore, the frequency of second surgery is higher in patients treated with local excision than in patients treated with radical resection. Mellgren et al[3] reported 17 cases (68%) of recurrent rectal cancer in the rectal wall and 8 cases (32%) of recurrent rectal cancer in extramural. Sengupta et al[4] found that 40%-100% of recurrent rectal cancer patients could be cured. Friel et al[17] used salvage therapy for 29 T1 or T2 tumor patients with recurrence after local excision (90% involving the rectal wall and 10% completely extramural), and found no residual tumor in 17 survivors after a mean follow-up time of 39 mo. Of the 18 patients with rectal tumor recurrence in our study, 12 were retreated surgically. Of the 5 patients treated with local excision, 1 died after 93 mo and 4 survived. In the 6 patients treated with abdominoperineal resection and 1 patient treated with Park’s procedure, 2 died after 36 and 40 mo, respectively, and the other 5 survived, suggesting that salvage therapy is effective against early recurrent rectal cancer.
In rectal cancer patients who are unsuitable for or refuse abdominoperineal resection, local excision can be performed after the tumor is down staged[17]. Kim et al[18] showed that preoperative adjuvant therapy can effectively down stage T2 and T3 rectal cancer. In their study, 25 patients who were staged before operation by endorectal ultrasound (ERUS) received radiotherapy (45 Gy, 25 cycles) and chemotherapy (5-flurouracil, 300 mg/m2 per day, 5 d/week). Partial pathological response was achieved in 4 patients and complete response in 22 patients. In the 22 patients with complete-response, no recurrent rectal cancer occurred after local excision. In our 4 patients with preoperative stage T3 rectal cancer treated with local excision after chemotherapy, the cancer recurred in 2 cases with a recurrence rate of 50%. Since T3 and T4 tumors have a higher recurrent rate, they are unsuitable for local excision in patients who cannot tolerate or refuse abdominoperineal resection.
Local rectal cancer can be treated with a variety of methods, including TAE, TSE, TVE, and minimally invasive endoscopic transanal excision[19]. Irrespective of the surgical method, R0 excision is the most important procedure. TAE, a minimally invasive treatment modality, has few complications[20]. In our 92 patients, TAE had a low complication rate of 2.2%. Preoperative pelvic CT and ultrasound examination can provide important information for surgical planning. Kwok et al[21] analyzed data on the accuracy, sensitivity, and specificity of preoperative CT, magnetic resonance imaging (MRI), MRI-endorectal coil (MRI-ERC), and ERUS for rectal cancer staging, and found that the accuracy of CT, MRI, MRI-ERC, and ERUS is 80%, 74%, 81%, and 84%, respectively, with a higher accuracy for T1 tumors. At present, local excision is a generally accepted treatment modality for stage Tis and T1 rectal cancer. However, close follow-up is required. Early detection and salvage therapy can prolong the survival time of rectal cancer patients.
Both abdominoperineal resection and anterior resection of rectal cancer can result in postoperative urinary impairment and sexual dysfunction, and the presence of a permanent colostomy significantly impacts the quality of life. With the advancement of imaging techniques, the accuracy of preoperative rectal cancer staging has increased dramatically, and the preservation of physical function in rectal cancer patients has become a very important aim of research. Local tumor excision preserves anal, urinary, and sexual function in patients with low rectal cancer. Therapeutic efficacy and surgical indications for this procedure are controversial.
Some studies have demonstrated that local tumor excision of early stage low rectal cancer can produce a good outcome and preserve anal, urinary, and reproductive function. However, a higher local recurrence rate (5%-27%) of this procedure has limited its use in clinical practice. Nevertheless, the 5-year survival rate after local tumor excision is 72%-95%. Risk factors affecting low rectal cancer prognosis after local excision include age and sex of patients, treatment modalities, and vascular invasion and tumor stage.
In this study, age and sex of the patients were unrelated to their prognosis. Vascular invasion and tumor stage affected rectal cancer recurrence and prognosis after local excision, and local recurrence and distance metastasis rates increased significantly when the tumor was poorly differentiated, macroscopically ulcerative, or larger than 3.0 cm in diameter. Therefore local excision should be recommended with caution for such patients. Frozen section examination of margins during surgery ensures margin negativity, which is essential for complete local tumor excision. Salvage therapy is of great importance in the treatment of recurrent rectal cancer after local excision.
Local rectal cancer excision has few complications, and is suitable for stages Tis and T1 rectal cancer. Prevention of local recurrence, active postoperative follow-up, and salvage therapy can greatly increase the efficacy of local excision.
Local rectal cancer can be treated with a variety of methods including transanal excision (TAE), transsacral excision (TSE), transvaginal excision (TVE), transsphincter local excision, and minimally invasive endoscopic transanal excision
The study is well designed and it results have confirmed the efficiency of different methods for rectal cancer and the discussion is reasonable.
| 1. | Heintz A, Mörschel M, Junginger T. Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc. 1998;12:1145-1148. |
| 2. | Willett CG, Compton CC, Shellito PC, Efird JT. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer. 1994;73:2716-2720. |
| 3. | Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064-1071; discussion 1071-1074. |
| 4. | Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345-1361. |
| 5. | Blair S, Ellenhorn JD. Transanal excision for low rectal cancers is curative in early-stage disease with favorable histology. Am Surg. 2000;66:817-820. |
| 6. | Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut. 1977;18:1045-1050. |
| 7. | Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773-1779. |
| 8. | Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, Nathanson DR, Guillem JG, Enker WE, Cohen AM. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522-529; discussion 529-530. |
| 9. | Steele GD Jr, Herndon JE, Bleday R, Russell A, Benson A 3rd, Hussain M, Burgess A, Tepper JE, Mayer RJ. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433-441. |
| 10. | Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360-363. |
| 11. | Bouvet M, Milas M, Giacco GG, Cleary KR, Janjan NA, Skibber JM. Predictors of recurrence after local excision and postoperative chemoradiation therapy of adenocarcinoma of the rectum. Ann Surg Oncol. 1999;6:26-32. |
| 12. | Adachi Y, Yasuda K, Kakisako K, Sato K, Shiraishi N, Kitano S. Histopathologic criteria for local excision of colorectal cancer: multivariate analysis. Ann Surg Oncol. 1999;6:385-388. |
| 13. | Sitzler PJ, Seow-Choen F, Ho YH, Leong AP. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum. 1997;40:1472-1476. |
| 14. | Rothenberger DA, Garcia-Aguilar J. Role of local excision in the treatment of rectal cancer. Semin Surg Oncol. 2000;19:367-375. |
| 15. | Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, Kaufman D, Ancukiewicz M, Willett CG. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49-54. |
| 16. | Lamont JP, McCarty TM, Digan RD, Jacobson R, Tulanon P, Lichliter WE. Should locally excised T1 rectal cancer receive adjuvant chemoradiation? Am J Surg. 2000;180:402-405; discussion 405-406. |
| 17. | Friel CM, Cromwell JW, Marra C, Madoff RD, Rothenberger DA, Garcia-Aguílar J. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875-879. |
| 18. | Kim CJ, Yeatman TJ, Coppola D, Trotti A, Williams B, Barthel JS, Dinwoodie W, Karl RC, Marcet J. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001;234:352-358; discussion 358-359. |
| 19. | Chorost MI, Petrelli NJ, McKenna M, Kraybill WG, Rodriguez-Bigas MA. Local excision of rectal carcinoma. Am Surg. 2001;67:774-779. |
| 20. | Visser BC, Varma MG, Welton ML. Local therapy for rectal cancer. Surg Oncol. 2001;10:61-69. |
| 21. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. |