Published online Mar 14, 2009. doi: 10.3748/wjg.15.1231
Revised: February 10, 2009
Accepted: February 17, 2009
Published online: March 14, 2009
AIM: To investigate the pathogenic role of cathepsin B and the protective effect of a cathepsin B inhibitor (CA-074Me) in fulminant hepatic failure in mice.
METHODS: LPS/D-Gal N was injected into mice of the model group to induce fulminant hepatic failure; the protected group was administered CA-074me for 30 min before LPS/D-Gal N treatment; the normal group was given isochoric physiologic saline. Liver tissue histopathology was determined with HE at 2, 4, 6 and 8 h after Lps/D-Gal injection. Hepatocyte apoptosis was examined by TUNEL method. The expression of cathepsin B in liver tissues was investigated by immunohistochemistry, Western blot and RT-PCR.
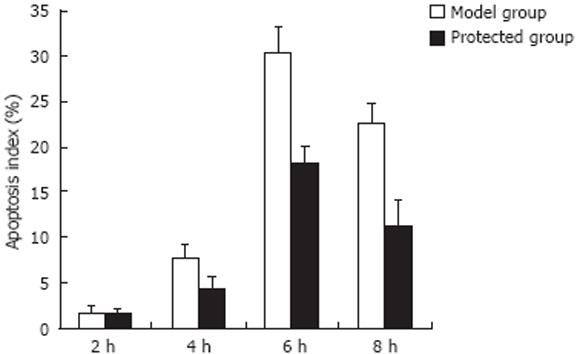
RESULTS: Compared with the normal group, massive typical hepatocyte apoptosis occurred in the model group; the number of apoptotic cells reached a maximum 6 h after injection. The apoptosis index (AI) in the protected group was clearly reduced (30.4 ± 2.8 vs 18.1 ± 2.0, P < 0.01 ). Cathepsin B activity was markedly increased in drug-treated mice compared with the normal group (P < 0.01). Incubation with LPS/D-Gal N at selected time points resulted in a time-dependent increase in cathepsin B activity, and reached a maximum by 8 h. The expression of cathepsin B was significantly decreased in the protected group (P < 0.01).
CONCLUSION: Cathepsin B plays an essential role in the pathogenesis of fulminant hepatic failure, and the cathepsin B inhibitor CA-074me can attenuate apoptosis and liver injury.
- Citation: Yan BZ, Wang W, Chen LY, Bi MR, Lu YJ, Li BX, Yang BS. Role of cathepsin B-mediated apoptosis in fulminant hepatic failure in mice. World J Gastroenterol 2009; 15(10): 1231-1236
- URL: https://www.wjgnet.com/1007-9327/full/v15/i10/1231.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1231
Fulminant hepatic failure is a rare, but severe, complication of acute hepatitis; it is associated with very high mortality. It has been reported that fulminant hepatic failure is an inflammatory process that causes the death of liver cells by necrosis or by triggering apoptosis[1–3]. Cathepsins are a family of proteolytic enzymes, many of which, including cathepsin B, are cysteine proteinases. Recent evidence suggests that cathepsin B contributes to cell apoptosis[45]. It is not known if cathepsin B-mediated hepatocyte apoptosis is involved in the pathogenesis of fulminant hepatic failure. The aim of the present study was to determine if fulminant hepatic failure contributes to a change in the expression of cathepsin B protein and mRNA. To ascertain its pathogenic role in hepatic failure, we examined the protective effect of a cathepsin B inhibitor, CA-074Me [N-(L-trans-propylcarbamoyloxirane-2-carbonyl)-L-isoleucyl-L-proline] on fulminant hepatic failure in mice.
Kunming mice (male, 18-20 g, 4 wk of age) were used. Animals were provided by the Animal Center of the First Clinical Hospital of Harbin Medical University. This study conformed to Harbin Medical University’s guidelines for the care and use of laboratory animals.
Seventy-two mice were randomized to three groups (normal control, model and protected). Galactosamine (D-Gal N) 800 mg/kg and lipopolysaccharide (LPS) 100 &mgr;g/kg were injected into the abdominal cavity of mice of the model group; mice in the protected group were administered CA-074me (10 mg/kg) for 30 min before LPS/D-Gal N treatment; and the normal control group was given isochoric physiologic saline. Six mice in each group were killed 2, 4, 6 and 8 h after injection. The liver was cut into small pieces and snap-frozen in liquid nitrogen and stored at -70°C, or fixed in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C.
Goat anti-mouse cathepsin B polyclonal IgG and P-conjugated secondary antibodies for immunoblotting were purchased from Santa Cruz Biotechnology Inc., USA; D-Gal N, LPS and CA-074me from Sigma (USA); TUNEL reagent kit from Zhong Shan Biotechnical Ltd (Beijing, China); immunohistochemical kit from Zymed (series SP kits); ABC reagent and DAB kits from Wuhan Boster Biological Technology Co, Ltd (Wuhan, China); Trizol kits from Invitrogen (USA); and the reverse transcription-polymerase chain reaction (RT-PCR) kit from Promega (USA). Primers were synthesized by Shanghai Sangon Biological Engineering Technology Co, Ltd (Shanghai, China).
The liver was fixed in 4% paraformaldehyde for 48 h, and embedded in paraffin. Tissue sections were prepared with a microtome and placed on glass slides. Hematoxylin and eosin staining was done by standard methods. TUNEL assay was carried out with a commercially available kit according to the manufacturer’s instructions. Hepatocyte apoptosis in liver sections was quantified by counting the number of TUNEL-positive cells in microscopic high-power fields.
Sections were incubated with goat anti-mice cathepsin B, which was pre-diluted by the manufacturer for staining formalin-fixed paraffin-embedded tissues. After washing the sections exhaustively, they were incubated for 45 min with biotin-conjugated anti-goat IgG antibody, and then with horseradish peroxidase (HRP)-conjugated streptavidin. Negative control slides were incubated with non-immune immunoglobulin under identical conditions. Liver cell endochylema or nucleus containing yellow granulation served as a positive control, followed by semi-quantitative analysis using Image-plus 6.0 software.
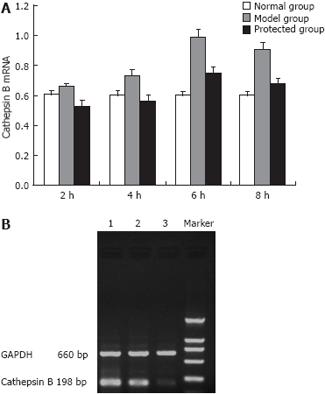
Total RNA was obtained from whole liver using trizol reagent. The RNA sample was reversely transcribed into cDNA according to manufacturer’s instructions. The primers for the experiment were as follows: cathepsin B[6], forward 5’-GAAGAAGCTGTGTGGCACTG-3’, and reverse 5’-GTTCGGTCAGAAATGGCTTC-3’ (yielding a 198-bp product); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5’-CTGCACCACCAACTGCTTAG-3’, and reverse 5’-GTCTGGGATGGAAATT GTGA-3’ (660-bp). GAPDH was used as a control for RNA integrity. Thermal cycling conditions were 15 seconds at 96°C, 62°C for 20 s, and 1 min at 70°C. Amplification was stopped after 34 cycles. Ten microliters of PCR products were obtained in each group, and confirmed by gel electrophoresis (coloration by EB, Ethidium Bromide). Gel electrophoresis photographs were taken; the band of Cathepsin B PCR products on electrophoresis gel were quantified using a DNA sequencer equipped with Quantity-One analysis software.
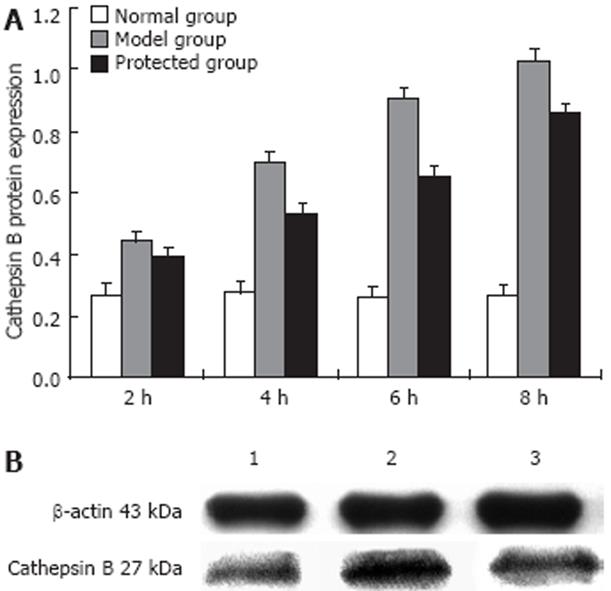
Samples were centrifuged at 10 000 ×g for 20 min at 4°C to remove solid material. Supernatants were centrifuged at 100 000 ×g for 1 h at 4°C. Cellular protein from each sample (50 &mgr;g) was mixed with sample buffer (0.25 mol/L Tris, pH 6.8, 8% SDS, 40% glycerol, 2.5% bromphenol blue, and 2% β-mercaptoethanol), heated for 3 min at 95°C, applied to a 12% acrylamide gel, separated by electrophoresis (SDS-polyacrylamide gel electrophoresis) and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h. Blots were incubated in cathepsin B polyclonal antibody at a dilution of 1: 200 for 2 h. After washed three times for 10 min with TBST, blots were incubated with HRP-conjugated anti-goat secondary antibody (1:5000) for 1 h. Following the secondary antibody incubations, blots were developed using an ECL-plus kit. Blots were visualized using the chemiluminescence detection system (Amersham Pharmaceuticals, Amersham, UK).
Data represent at least four independent experiments, and were expressed as the mean ± SD (unless otherwise indicated). The difference was determined using two-way analyses of variance (ANOVA) following SNK. Data were analyzed by SPSS software. P < 0.05 was considered to be statistically significant.
Liver histopathology was used to assess if different groups had different effects on liver injury (Figure 1). Liver histology was normal in the control group. After treatment with D-Gal N and LPS for 6 h, massive hepatocyte apoptosis was detected in the model group; 8 h after injection, hepatocyte necrosis with massive infiltrates of neutrophils was widely spread. The protected group had far less apoptosis and necrosis, and little evidence of neutrophil accumulation, especially 6 and 8 h after injection.
Hepatocyte apoptosis was quantified using TUNEL assay (see Materials and Methods). None or few TUNEL-positive cells were observed in the normal group. In the model group, massive hepatocyte apoptosis occurred, and the number of apoptotic cells increased with time (Figure 2). The apoptosis index (AI) 2 h after injection of D-Gal N/LPS was (1.8 ± 0.7)%, (7.8 ± 1.5)% at 4 h, and reached a climax at 6 h (30.4 ± 2.8)%. After 8-h treatment, AI was lower than that of 6 h, and reached (22.6 ± 2.2)% at 8 h. Compared with the model group, the apoptotic cells in the protected group were obviously reduced at the same time points, particularly at 6 h (18.1 ± 2.0)% and 8 h (11.4 ± 2.6)% (P < 0.01).
Immunohistochemistry for cathepsin B was done to confirm apoptosis (Figure 3). Strongly positive immunoreactivity for active cathepsin B was detected in mice injected with LPS/D-Gal N compared with the normal group. After 6-h and 8-h treatment with LPS/D-Gal N, more expression of cathepsin B was found (0.251 ± 0.010 and 0.280 ± 0.011, respectively). At the same time points, cathepsin B was found to be significantly less expressed in mice of the protected group (0.202 ± 0.008 and 0.241 ± 0.011, respectively, P < 0.01).
Semi-quantitative RT-PCR was used to assess the expression of cathepsin B mRNA. Using densitometric analysis in comparison with the housekeeping gene GADPH, we found that the expression of cathepsin B mRNA significantly increased after treatment of LPS/D-Gal N compared with the normal group (P < 0.01). The expression of cathepsin B mRNA gradually increased 6 h and 8 h after injection with LPS/D-Gal N and reached a climax at 6 h. At the same time points, expression of cathepsin B mRNA in the protected group was significantly reduced compared with the model group, particularly at 6 h and 8 h (Figure 4).
Western blot analysis of cathepsin B protein expression is shown in Figure 5. A prominent band at a molecular size of about 27 kDa was detected, which represented the single-chain form of cathepsin B. Incubation with LPS/D-Gal N at selected time points resulted in a time-dependent increase in cathepsin B protein. The cathepsin B protein began to increase 2 h after treatment with LPS/D-Gal N and increased to maximum activity at 8 h compared with the normal group (P < 0.01). Expression of cathepsin B was significantly decreased in the protected group, particularly at 8 h.
The traditional view is that hepatocyte necrosis is the main feature of fulminant hepatic failure, but increasing evidence implicates a dominant role for hepatocyte apoptosis in this pathogenesis[17]. The major objective of our study was to evaluate apoptosis-mediated fulminant hepatic failure in LPS /D-Gal N-induced liver injury.
Cathepsin B, a lysosomal cysteine protease, is a candidate for an apoptotic mediator originating from acidic vesicles. Cathepsin B is synthesized as a 38-kDa procathepsin B that undergoes sequential processing steps within lysosomes. First, a 30-kDa mature active form is generated by proteolytic cleavage[8]. Further processing involves removal of the NH2-terminal propeptide, cleavage of six residues from the COOH-terminus, and internal excision of residues 127 and 128 to generate a two-chain (a 27-kDa heavy chain and a 4-5-kDa light chain) form of the enzyme with inter-chain disulfide bonds. Pharmacologic inhibition of cathepsin B has been reported to block apoptosis induced by p53 and cytotoxic agents[9]. Recent evidence suggests that cathepsin B contributes to tumor necrosis factor- (TNF-α)-induced apoptosis in vitro and in vivo[1011]. In cell culture systems, activation of caspase 8 is associated with the release of cathepsin B from acidic vesicles into the cytosol. In the cytosol, cathepsin B was found to induce mitochondrial release of cytochrome C[1213] and activate caspase 9 and 3[14]. The importance of this pathway in TNF-α-mediated apoptosis in vitro was shown by demonstrating that hepatocytes isolated from cathepsin B knockout mice are resistant to TNF-α-induced apoptosis. More recently, some colleagues have demonstrated a dominant role for cathepsin B in TNF-α-mediated apoptosis. In a murine tumor cell line, caspase inhibition accentuated TNF-α-induced apoptosis by a cathepsin-B pathway. Inactivation of cathepsin B attenuates hepatocyte apoptosis and liver damage in liver reperfusion injury[1516] and cholestasis injury[17]. CA-074me is a selective inhibitor of cathepsin B[18]; it is highly cell-permeant and can decrease the expression or activity of cathepsin B. During the early stages of pancreatitis, trypsinogen activation in the pancreas is mediated by cathepsin B, indicating that pharmacological interventions that inhibit cathepsin B may prove useful in preventing acute pancreatitis or reducing its severity[19].
In summary, our studies suggested a role for cathepsin B in fulminant hepatic failure. Compared with the normal group, massive hepatocyte apoptosis occurred in the model group, and the number of apoptotic cells increased to a maximum at 6 h. Incubation for longer periods did not lead to further increase in apoptosis and instead resulted in an increase of cell necrosis. The apoptosis index in the protected group was obviously reduced. Consistent with these data, the activity of cathepsin B was markedly increased in drug-treated mice compared with the normal group. Incubation with LPS/D-Gal N for selected time points resulted in a time-dependent increase in cathepsin B activity, and reached a maximum at 8 h. Cathepsin B expression was significantly decreased in the protected group. Collectively, these data suggest that LPS/D-Gal N-mediated cathepsin B expression initiates hepatocyte apoptosis in fulminant hepatic failure. Inhibition of cathepsin B attenuates apoptosis and liver injury, supporting a link between cathepsin B and fulminant hepatic failure. Inhibition of hepatocyte apoptosis with CA-074me seems to be a feasible therapeutic option for fulminant hepatic failure.
Fulminant hepatic failure is a rare, but severe, complication of acute hepatitis; it is associated with very high mortality. The traditional view is that hepatocyte necrosis is the main feature of fulminant hepatic failure, but increasing evidence implicates a dominant role for hepatocyte apoptosis in this pathogenesis. Recent evidence suggests that cathepsin B contributes to cell apoptosis.
It is not known if cathepsin B-mediated hepatocyte apoptosis is involved in the pathogenesis of fulminant hepatic failure.
The current study demonstrated that cathepsin B has an essential role in the pathogenesis of fulminant hepatic failure, and the cathepsin B inhibitor CA-074me could attenuate apoptosis and liver injury.
Inhibition of cathepsin B attenuates apoptosis and liver injury, and CA-074me seems to be a viable therapeutic option for fulminant hepatic failure.
Cathepsin B, a lysosomal cysteine protease, is a candidate for an apoptotic mediator originating from acidic vesicles. CA-074me is a specific inhibitor of cathepsin B; it is highly cell-permeant and can decrease the expression or activity of cathepsin B.
This is a well-designed, most part well-written paper, with important new data on the pathogenesis of fulminant hepatic failure. Authors have shown that apoptosis is important in the development and that the process can be partially reversed by specific cathepsin-B inhibitor.
| 1. | Kuhla A, Eipel C, Siebert N, Abshagen K, Menger MD, Vollmar B. Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis. 2008;13:1427-1438. |
| 2. | Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85-89. |
| 3. | Doggrell SA. Suramin: potential in acute liver failure. Expert Opin Investig Drugs. 2004;13:1361-1363. |
| 4. | Houseweart MK, Vilaythong A, Yin XM, Turk B, Noebels JL, Myers RM. Apoptosis caused by cathepsins does not require Bid signaling in an in vivo model of progressive myoclonus epilepsy (EPM1). Cell Death Differ. 2003;10:1329-1335. |
| 6. | Dong Z, Katar M, Linebaugh BE, Sloane BF, Berk RS. Expression of cathepsins B, D and L in mouse corneas infected with Pseudomonas aeruginosa. Eur J Biochem. 2001;268:6408-6416. |
| 7. | Kasahara I, Saitoh K, Nakamura K. Apoptosis in acute hepatic failure: histopathological study of human liver tissue using the tunel method and immunohistochemistry. J Med Dent Sci. 2000;47:167-175. |
| 8. | Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629-4633. |
| 9. | Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol. 2000;62:273-295. |
| 10. | Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127-1137. |
| 11. | Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol. 2001;159:2045-2054. |
| 12. | Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149-3157. |
| 13. | Cirman T, Oresić K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279:3578-3587. |
| 15. | Baskin-Bey ES, Canbay A, Bronk SF, Werneburg N, Guicciardi ME, Nyberg SL, Gores GJ. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2005;288:G396-G402. |
| 16. | Ben-Ari Z, Mor E, Azarov D, Sulkes J, Tor R, Cheporko Y, Hochhauser E, Pappo O. Cathepsin B inactivation attenuates the apoptotic injury induced by ischemia/reperfusion of mouse liver. Apoptosis. 2005;10:1261-1269. |
| 17. | Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, Gores GJ. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152-159. |
| 18. | Linebaugh BE, Sameni M, Day NA, Sloane BF, Keppler D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur J Biochem. 1999;264:100-109. |
| 19. | Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794-G800. |