INTRODUCTION
Patients with both ulcerative colitis (UC) and Crohn’s disease (CD) are at an increased risk of developing colorectal cancer (CRC). It is believed that this increased risk is a result of persistent inflammation of the colon. The exact mechanism as to how chronic inflammation results in carcinogenesis is unclear, but it is postulated that the same genetic mutations that result in sporadic CRC are also responsible for its development in inflammatory bowel disease (IBD). Surveillance guidelines employing colonoscopy as a tool for screening this high-risk population are available. Unfortunately, the guidelines have their limitations. Additionally, no consensus exists regarding the management of low-grade dysplasia (LGD) in the setting of IBD. This article will review the CRC risk factors in IBD, current surveillance guidelines, management of dysplasia in IBD, and lastly chemoprevention.
EPIDEMIOLOGY OF CRC IN PATIENTS WITH IBD
It has been known for nearly a century that UC is associated with an increased risk of CRC[12]. However, the association between CRC and CD has only recently been recognized[3–5]. The mean duration from the time of diagnosis of UC to the development of CRC is 17 years, with the mean age at diagnosis of CRC being 51 years in men and 54 years in women[6]. In a large meta-analysis involving 54 478 patients and 116 studies, Eaden et al[7] calculated the cumulative risk of CRC in UC at 8.3% at 20 years and 18.4% at 30 years. These results, however, have not been replicated, with more recent studies showing a lower annual incidence (0.06%-0.2%) of CRC in patients with UC[89]. Interestingly, two separate population-based studies from Denmark and the Mayo Clinic found no increased risk between CRC and UC when compared to the general population[1011]. The differences among these studies have been postulated to be the result of improved medical therapies with unforeseen chemoprevention, increased colonoscopic surveillance, and improved surgical treatments.
Although there is less data regarding the risk of CRC in CD, it is well established that a similar association exists to that of UC[3–5]. However, the development of dysplasia in CD can involve both the small bowel as well as the colon. In a population-based study from the Mayo Clinic, only a slight increased risk was found between CD and CRC, which is in stark contrast to a 40-fold increased risk of developing small bowel malignancy[10]. Interestingly, an earlier large population-based study from Sweden reported a relative risk of 5.6 for the development of CRC in patients with Crohn’s colitis[3].
PATHOGENESIS OF CRC IN PATIENTS WITH IBD
The exact mechanism by which chronic inflammation results in carcinogenesis is unclear. Persistent inflammation is believed to result in increased cell proliferation as well as oxidative stress, and ultimately the development of dysplasia[12–1415]. It is postulated that similar genetic mutations that result in sporadic CRC in the general population are also responsible for its development in IBD, but the sequence of events and frequency are altered[1617]. These events include microsatellite instability, inhibition of regulatory genes via hypermethylation of the promoter regions, and loss of adenomatous polyposis coli (APC), p53, and K-ras tumor suppressor function[1218]. In sporadic CRC among the general population, loss of APC function generally occurs early and is frequent, whereas p53 mutations occur late and are less frequent. In contrast, loss of APC function generally occurs late and is infrequent, whereas p53 mutations occur early and are more frequent in IBD-associated CRC[18].
RISK FACTORS FOR CRC IN PATIENTS WITH IBD
Multiple risk factors for the development of CRC in the setting of IBD have been identified. It is well established that greater disease duration and anatomic extent of colitis are important risk factors[19]. Generally, patients with pancolitis develop CRC a decade prior to patients with left-sided colitis. Of note, disease extent is defined as the furthest extent of inflammation, either microscopically or macroscopically[20]. Recent studies have also demonstrated that the degree of colonic inflammation is associated with an increased risk of dysplasia and CRC. This was first introduced by the group at St. Mark’s Hospital[21], and further confirmed by two recent studies[2223]. Additionally, other markers of inflammation, including the presence of pseudopolyps, strictures, and backwash ileitis have been found to be independently associated with an increased risk of developing CRC[24–26].
Independent of a family history of IBD, a family history of sporadic CRC imparts a two-fold higher risk of CRC in IBD patients[2728]. Also, IBD diagnosis at an earlier age increases the risk of CRC, independent of disease duration[19]. In fact, a Swedish population-based cohort of 3117 patients diagnosed with UC between 1922 and 1983, found a four-fold increase in CRC in patients diagnosed with UC prior to the age of 15 compared with those diagnosed between 15-29 years of age[19]. Lastly, the concomitant presence of primary sclerosing cholangitis (PSC) confers a significantly increased risk of CRC in patients with UC[29]. Among patients with PSC, Kornfeld et al[30] calculated a cumulative risk of developing CRC of 33% at 20 years and 40% at 30 years after the diagnosis of UC.
DEVELOPMENT OF DYSPLASIA IN IBD
Unlike sporadic CRC in the general population, the development of carcinogenesis in IBD does not always follow a sequential progression from LGD, to high-grade dysplasia (HGD), and ultimately to cancer[18]. In fact, Ullman et al[31] revealed that cancer can arise in patients with no prior dysplasia, or without first progressing from LGD to HGD. Additionally, they found that patients undergoing colectomy for flat LGD were found to have much more advanced pathology on surgical specimens. Also, CRC arising in the background of IBD is often multifocal and more aggressive than sporadic CRC[32–34]. This unpredictable sequence of events coupled with its more aggressive nature highlights the importance of increased vigilance and surveillance for CRC in this high-risk population.
Dysplasia is defined as the unequivocal neoplastic alteration of the epithelium without invasion into the lamina propria[35]. Macroscopically, dysplastic lesions in the setting of colitis can range from flat lesions (not endoscopically visible) to plaque-like lesions, to raised localized or multifocal lesions[3637]. Raised, endoscopically visible lesions noted within areas of active colitis are referred to as dysplasia-associated lesions or masses (DALMs)[36]. These are further categorized into adenoma-like lesions and non-adenoma-like lesions, which ultimately results in different management implications[38]. Microscopically, per the IBD Dysplasia Morphology Study Group, dysplasia is divided into three categories: (1) negative for dysplasia; (2) indefinite for dysplasia; and (3) positive for dysplasia, which is further divided into LGD and HGD[35]. Unfortunately, inter-observer agreement and intra-observer reliability among pathologists is variable in the diagnosis of dysplasia, especially when diagnosing indeterminate and LGD[3940]. Therefore, it is traditionally recommended that any diagnosis of dysplasia be confirmed by a separate GI pathologist[41].
CRC SURVEILLANCE IN IBD
Recommendations for CRC screening/surveillance in IBD is aimed at early detection and mortality reduction from CRC. Although a multitude of studies exist demonstrating the need for secondary prevention[42–45], a 2004 Cochrane Database review did not show “clear evidence that surveillance colonoscopy prolonged survival.” However, this analysis indirectly revealed a reduced mortality risk in IBD-associated CRC[46].
The most recent surveillance strategy, published in 2005 by the Crohn’s and Colitis Foundation of America (CCFA), is based on expert consensus of past surveillance guidelines as well as more recent studies on IBD-associated dysplasia[41]. Per the consensus guidelines, screening colonoscopies should begin 8-10 years after the onset of IBD symptoms in patients with UC pancolitis/left-sided colitis and Crohn’s colitis involving at least one third of the colon. The exception to this rule is that patients with concomitant PSC begin yearly surveillance colonoscopies at the time PSC is diagnosed. The timing of follow-up surveillance colonoscopies depends on the presence of dysplasia. If the initial colonoscopy in UC pancolitis/left-sided colitis (and Crohn’s colitis) is negative for dysplasia, repeat surveillance colonoscopy should be performed in 1-2 years. Once a patient has two negative surveillance colonoscopies, further surveillance colonoscopies should be performed every 1-3 years. After 20 years of disease duration, surveillance colonoscopies should be repeated every 1-2 years. Management of dysplasia is described below. The recommended surveillance biopsies are four-quadrant biopsies every 10 cm with jumbo forceps. Of note, patients with only proctosigmoiditis of < 35 cm disease extent should follow the standard CRC screening guidelines for the general population, as they are not at an increased risk of developing CRC[41].
MANAGEMENT OF DYSPLASIA
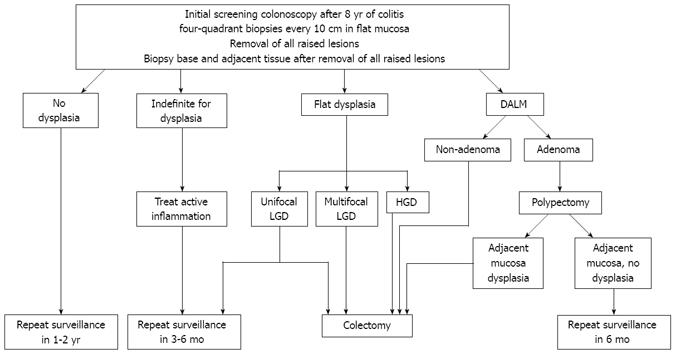
All dysplastic and indefinite-for-dysplasia biopsies should be confirmed by an expert GI pathologist. Once confirmed, patients with HGD or multifocal flat LGD should be referred for prophylactic total proctocolectomy. Patients with indefinite dysplasia should undergo a more aggressive surveillance plan, with repeat surveillance in 3-6 mo. Controversy exists regarding the management of unifocal flat LGD, as studies have calculated a wide progression rate of 2%-50% from LGD to HGD[3147–49]. Therefore, it is imperative that an open dialogue regarding the risks and benefits of both surgical and intense colonoscopic surveillance (repeat surveillance colonoscopies every 3-6 mo) be discussed with all patients found to have unifocal flat LGD. If intense surveillance is chosen by the patient, the CCFA consensus strongly recommends a proctocolectomy if multifocal flat LGD, repetitive flat LGD, or more advanced dysplasia is found during subsequent surveillance colonoscopies[41].
All non-adenoma-like DALMs should be referred for a complete proctocolectomy, as up to 50% of these patients can have cancer at the time of surgery[3550]. Per the CCFA consensus guidelines, adenoma-like DALMs should be resected in their entirety, with the base and surrounding mucosa biopsied separately. Patients should be referred for surgery if the base or surrounding mucosa contains dysplasia. However, if no dysplasia is found in the base or surrounding mucosa, a repeat colonoscopy should be performed in 6 mo[41]. A summary of currently accepted algorithm for surveillance of CRC and management of dysplasia is presented in Figure 1.
Figure 1 Algorithm for screening/surveillance for CRC in IBD and management of dysplasia.
CRC: Colorectal cancer; IBD: Inflammatory bowel disease; LGD: Low grade dysplasia; HGD: High grade dysplasia; DALM: Dysplasia-associated lesions or masses.
Lastly, special attention should be applied to all colonic strictures in IBD. In UC patients, strictures should prompt surgical referral, given the high association with underlying malignancy[41]. Although the risk of malignancy is not as high in Crohn’s-related colonic strictures, efforts should be made to visualize the colon proximal to the stricture, since an increased risk of CRC does exist. In fact, a 6.8% frequency rate of colon cancer after 20 years disease duration was found among a review of 175 Crohn’s colon strictures[51]. Surgical resection should therefore be considered in longstanding CD, or if the colon proximal to the stricture cannot be visualized[4151].
Although guidelines are available for CRC surveillance in IBD, multiple limitations exist. Per the current four-quadrant biopsies every 10 cm guideline, only less than 1% of the entire mucosal surface of the colon is sampled, leaving a very high sampling error[18]. Additionally, pathologists do not universally agree on the diagnosis of low-grade or indefinite-for-dysplasia, especially in a field of active inflammation[3940]. Lastly, the management of flat LGD is often debated, specifically whether to proceed directly to surgery or follow a more aggressive colonoscopic surveillance plan.
CHEMOPREVENTION
The use of pharmacotherapy as a potential chemopreventive measure to reduce the risk of developing CRC has been studied extensively. The most widely studied agent for chemoprevention is 5-aminosalicylate (5-ASA). Unfortunately, no prospective studies evaluating 5-ASA as a chemopreventive agent exist, and the data is otherwise conflicting. The most compelling data from a meta-analysis of nine observational studies demonstrated a reduced risk of developing CRC or dysplasia with 5-ASA (OR = 0.51, 95% CI 0.38-0.69)[52]. This is in contrast to a retrospective case-controlled study of 25 patients with IBD and CRC, which demonstrated no association between 5-ASA use and reduction in CRC risk[53]. Nonetheless, with its relatively low side-effect profile, 5-ASA appears to be protective against CRC in IBD.
Additional agents studied for their chemopreventive effects include ursodeoxycholic acid (UDCA), corticosteroids, NSAIDs, folate, statins, calcium, and immunomodulators[18]. Two separate studies have demonstrated that the use of UDCA in patients with concomitant PSC and UC lowers the incidence of developing dysplasia or CRC (OR = 0.18 and 0.26, respectively)[5455]. Thus, the use of UDCA for chemoprevention in patients with both UC and PSC is to be encouraged. However, the role of UDCA as a chemopreventive agent in UC without PSC is unknown. Currently, insufficient data or inadequate evidence precludes the use of folate, corticosteroids, NSAIDs, calcium, statins, and immunomodulators as chemoprotective agents against CRC[2456–59].
ADVANCES IN DYSPLASIA DETECTION
As a result of the difficulty in identifying flat dysplastic lesions, the use of magnification chromoendoscopy was introduced. Magnification chromoendoscopy enhances mucosal contrast via the application of stains/dyes. Ideally, this would highlight mucosal changes that would otherwise not be seen by traditional white light endoscopy[60]. Methylene blue and indigo carmine are the most commonly studied dyes in UC surveillance[6162]. In addition to stains/dyes, advances in imaging technique, including narrow band imaging and confocal laser endomicroscopy may further enhance the ability to target abnormal areas of the colonic mucosa, potentially even at the subcellular level. Currently, data on these new techniques are limited, and are not included, at this time, in surveillance guidelines[63].
CONCLUSION
The risk of CRC in long-standing UC and CD has resulted in the adoption of surveillance strategies with the goals of reducing morbidity and mortality associated with IBD-related CRC. Risk factors for the development of CRC in the setting of IBD include disease duration, anatomic extent of disease, age at time of diagnosis, severity of inflammation, family history of colon cancer, and concomitant PSC. Although guidelines currently exist, limitations of these guidelines, including sampling error at time of biopsy, interobserver disagreement in histologically grading dysplasia, and disagreement about the management of LGD indicate the need for continued research into the molecular pathogenesis of IBD-associated CRC, with the hope of identifying targets for prevention. Advances in endoscopic imaging techniques are already underway, and may potentially aid in dysplasia detection and improve overall surveillance outcomes. Management of dysplasia depends predominantly on the degree and focality of dysplasia, with the mainstay of management involving either proctocolectomy or continued colonoscopic surveillance. Lastly, continued research into additional chemopreventive agents may increase our arsenal in attempting to reduce the incidence of IBD-associated CRC.
Peer reviewer: Rupert Leong, Associate Professor, Director of Endoscopy, Concord Hospital, ACE Unit, Level 1 West, Hospital Rd, Concord NSW 2139, Australia