Published online Mar 7, 2008. doi: 10.3748/wjg.14.1430
Revised: October 30, 2007
Published online: March 7, 2008
AIM: To determine the safety and efficacy of efficacy of percutaneous cryosurgery for treatment of patients with hepatic colorectal metastases.
METHODS: Three hundred and twenty-six patients with non-resectable hepatic colorectal metastases underwent percutaneous cryosurgery under the guidance of ultrasound or CT. Follow-up was 1 mo after cryosurgery and then every 4 mo thereafter by assessment of tumor markers, liver ultrasonography, and abdominal CT. For lesions suspicious of recurrence, a liver biopsy was performed and subsequent repeat cryosurgery was given if histology was positive for cancer.
RESULTS: All patients underwent a total of 526 procedures of cryosurgery. There were 151 patients who underwent repeat procedures of cryosurgery for recurrent tumors in the liver and extrahepatic places. At 3 mo after cryosurgery, carcinoembryonic antigen (CEA) levels in 197 (77.5%) patients who had elevated markers before cryosurgery decreased to normal range. Among 280 patients who received CT following-up, cryotreated lesions showed complete response (CR) in 41 patients (14.6%), partial response (PR) in 115 patients (41.1%), stable disease (SD) in 68 patients (24.3%) and progressive disease (PD) in 56 patients (20%). The recurrence rate was 47.2% during a median follow-up of 32 mo (range, 7-61). Sixty one percent of the recurrences were seen in liver only and 13.9% in liver and extrahepatic areas. The recurrence rate at cryotreated site was only 6.4% for all cases. During a median follow-up of 36 mo (7-62 mo), the median survival of all patient was 29 mo (range 3-62 mo). Overall survival was 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively, after the treatment. Patients with tumor size less than 3 cm, tumor in right lobe of liver, lower CEA levels (< 100 ng/dL) and post-cryosurgery TACE had higher survival rate. There was no significant difference in terms of survival based on the number of tumors, pre-cryosurgery chemotherapy and the timing of the development of metastases (synchronous vs metachronous). Patients who underwent 2-3 procedures of cryosurgery had increased survival compared to patients who received cryosurgery once only. There was no intra-cryosurgery mortality. Main adverse effects, such as hepatic bleeding, cryoshock, biliary fistula, liver failure, renal insufficiency and liver abscess were only observed in 0.3%-1.5% of patients.
CONCLUSION: Percutaneous cryosurgery was a safe modality for hepatic colorectal metastases. Rather than an alternative to resection, this technique should be regarded as a complement to hepatectomy and as an additional means of achieving tumor eradication when total excision is not possible.
- Citation: Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 2008; 14(9): 1430-1436
- URL: https://www.wjgnet.com/1007-9327/full/v14/i9/1430.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1430
Hepatic metastasis is the main cause of death in patients with colorectal carcinoma. Hepatic resection is the treatment of choice for liver cancer if metastases are confined to the liver, and may achieve a 5 years survival of 25%-39%[1–4]. Resectability is usually determined by the absence of extrahepatic metastases, a maximum of four lesions in the liver and the ability to obtain cancer-free resection margins[5].Therefore, only 10%-20% of patients with hepatic colorectal metastases are suitable for resection[6]. For non-resectable liver metastases, chemotherapy or chemoembolization are often used, but the outcome is poor. Median survival is around 12 mo for non-resectable hepatic colorectal metastases[7].
Cryosurgery has recently been applied to non-resectable liver tumors, and has shown encouraging results[8–11]. Between March 2001 and February 2007, 1090 patients with malignant liver tumors were treated by cryosurgery in our hospital. There were 680 patients with hepatocellular carcinoma (HCC), 326 with liver metastases originating from colorectal carcinoma, and 84 with liver metastases from cancer of non-colorectal origin. This study describes the results of percutaneous cryosurgery for the treatment of non-resectable hepatic colorectal metastases, with the purpose of determining the efficacy and safety of this modality.
Three hundred and twenty six patients with hepatic colorectal metastases were enrolled in this study. There were 243 men and 83 women with a mean age of 54.8 years (range, 32-84). Patient and tumor characteristics are listed in Table 1.
| Total cases | 326 |
| Median age (range) | 54 (32-84) |
| Male/Female (cases) | 243/83 |
| No. of tumors (cases) | |
| 1 | 125 (38.3%) |
| 2 | 105 (32.2%) |
| 3 | 65 (19.9%) |
| More | 31 (9.5%) |
| Tumor size (cases) | |
| < 3 cm | 95 (29.1%) |
| 3-5 cm | 124 (38.0%) |
| > 5 cm | 107 (32.8%) |
| Development of metastases | |
| Synchronous (cases) | 65 (19.9%) |
| Metachronous (cases) | 261 (80.1%) |
| Colorectomy to detection of metastases (mo) | 12 (0-42) |
| Metastases detected to cryosurgery (mo) | 4 (1-14) |
| Colorectomy to cryosurgery (mo) | 16 (3-52) |
| Precryosurgery chemotherapy(cases) | 216 (66.3%) |
| Precryosurgery CEA (mg/dL) | 11.2 (0.3-1422) |
Diagnosis of hepatic colorectal metastases was made by intraoperative findings during colorectomy, and in postoperative follow-up, by the combination of increased levels of tumor markers [carcinoembryonic antigen (CEA)] and imaging of lesions by ultrasonography, CT or MRI of the liver. There were 234 patients whose diagnosis was proven by liver biopsy. Liver metastases were synchronous in 65 cases (19.9%) and metachronous in 261 cases (80.1%). The tumors of all cases received a thorough investigation with regard to the presence of multiple nodules or to the presence of a large and/or ill-located tumor, comorbidity, and were considered as non-resectable. Patients with extrahepatic metastases or liver failure were excluded from this study.
All patients were given cryosurgery guidelines, and the study received ethical approval.
Cryosurgery was performed with Cryocare Operative System (Endocare, CA, USA) which used the Joule-Thomson effect to cool the end of a cryoprobe in closed systems. In accordance with the gas coefficient and the dimension of the nozzle, different gaseous elements generate different thermal exchange events at the area close to the nozzle. Argon gas is used for cooling (-187°C), and helium is used for heating (67°C). The probe was inserted percutaneously under ultrasound or CT guidance, and two freezing-thawing cycles were performed. We used mainly 2- or 5-mm probes and rarely a 10-mm probe, according to the size of the tumor. Two or more probes were used simultaneously for large lesions. Individual tumors may be frozen sequentially on a tumor-by-tumor basis or simultaneously. The time of freezing was dependent on the achievement of an "ice-ball", visible as a hypoechogenic area on ultrasonography, > 1 cm the diameter of the lesion. Thawing was achieved by input of helium during a period equivalent to the freezing time before the second freezing process was begun. Hemostasis of the insertion hole of the cryoprobe was obtained by Spongel application to the tract of the cryoprobe and by suture of the insertion site.
After cryosurgery, 280 patients underwent one or two sessions of TACE within 1-2 mo. The reasons for using TACE were larger tumors prior to cryosurgery, multiple tumors, or higher CEA level after cryosurgery. The chemotherapeutic agents for intra-arterial infusion were a mixture of Lipiodol and doxorubicin, cisplatinum, 5-fluorouracil, and mitomycin C. Occasionally gelfoam was used as an embolization material.
Postoperative follow-up was at the first month and then every 4 mo after cryosurgery, by assessment of liver function tests, tumor markers, liver ultrasonography, and abdominal CT. Some of patients received follow-up with positron emission tomography (PET). Efficacy of cryosurgery for tumors was evaluated according to the evolution of tumor size and tumor markers. Changes in tumor mass were measured according to the Response Evaluation Criteria in Solid Tumors (RECIST) protocol[12], which is based on objective measurement of lesion size before and after treatment. Complete response (CR) means cryotreated lesion disappearance (scar) or < 25% of original size. Partial response (PR) means a > 30% decrease in the sum of the largest diameter of all targeted lesions. Stable disease (SD) means < 30% decrease in the sum of the largest diameter of all targeted lesions. Progressive disease (PD) means an increase of > 20% in the sum of the largest diameter of all targeted lesions.
All radiologic studies were reviewed by the same radiologist with expertise in hepatic imaging. For lesions suspicious of recurrence, an ultrasound-guided liver biopsy was performed for histological study. Subsequent repeat cryosurgery was performed if histology was positive for cancer. A persistent nodule on radiological imaging, without tumor activity shown on PET, or with reduced or normal tumor markers (CEA), or no changes of the nodule size within at least 6 mo since cryosurgery, was considered as a remnant. Tumor recurrence was estimated either by histological examination of the liver, or by combination of size increase of the cryotreated lesion on ultrasound, CT or PET imaging and increased tumor markers.
Survival was determined according to the Kaplan-Meier method. Comparison of survival rates was obtained with the log-rank test. P < 0.05 was considered statistically significant.
All patients underwent a total of 526 procedures of cryosurgery. There were 175 patients who received a single procedure of cryosurgery, and 151 patients who underwent repeat procedures of cryosurgery for recurrent tumors in the liver and extrahepatic places (Table 2).
| Cryosurgery sites | No. of patients | Procedures of repeat cryosurgery |
| Liver only | 105 | 142 |
| Liver and lungs | 40 | 52 |
| Liver and pancreas | 6 | 6 |
| Total | 151 | 200 |
Increased CEA level was observed in 254 patients (77.9%) at the time of the initial diagnosis. Among these patients, CEA level decreased to within the normal range in 197 (77.5%) patients, and increased in 41 patients (16.1%), with no significant change in 16 cases (6.3%), at 3 mo after cryosurgery.
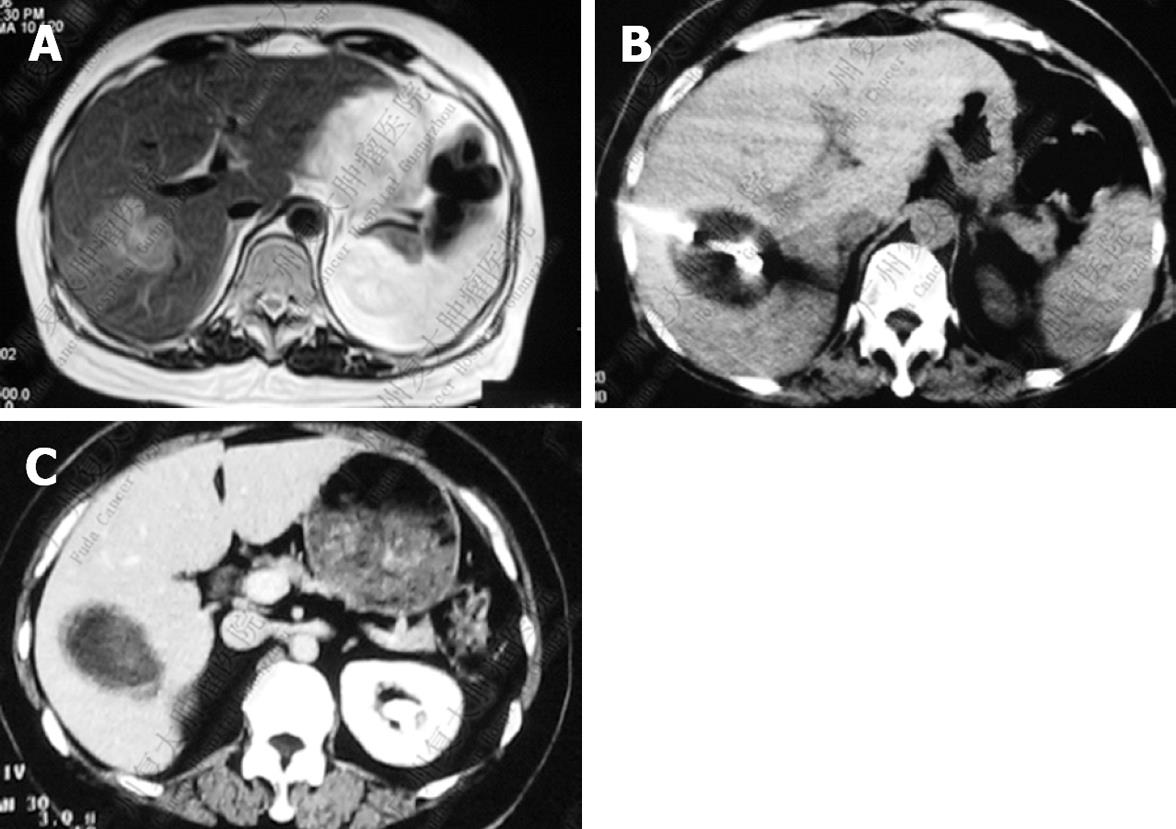
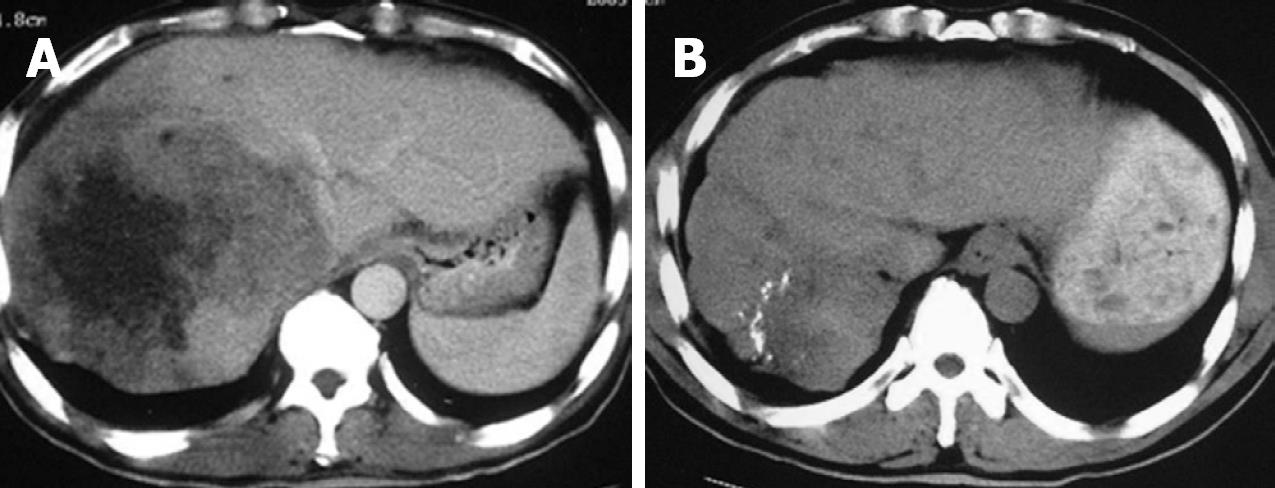
After cryosurgery, an early increase in the size of lesions in relation to the freezing margin > 1 cm beyond the limit of the tumor was a constant feature. Cryotreated lesions appeared as hypoechogenic or hypodense areas. Among 280 patients who received CT follow-up, CR was observed in 41 patients (14.6%), PR in 115 (41.1%), SD in 68 (24.3%), and PD in 56 patients (20%). Two patients with CR proven by histology and CT are presented in Figures 1 and 2.
The recurrence rate was 47.2% during a median follow-up of 32 mo (range, 7-61). Recurrence patterns are presented in Table 3. Sixty-one percent of recurrence was in the liver only and 13.9% in the liver and extrahepatic areas. Extrahepatic recurrence was mainly seen in the lungs and lymph nodes. The recurrence at cryotreated site, including at cryotreated site only as well as cryotreated site and the remaining area of liver, accounted 15.3% of cases who had recurrence and 6.4% of all cases.
| Cases n (%) | % of all cases | |
| Total recurrence | 136 (100) | 41.7 |
| Liver only | 83 (61.0) | 25.5 |
| Cryosite only | 7 (5.1) | 2.1 |
| Liver other than cryosite only | 62 (45.5) | 19.0 |
| Cryosite and remaining areas | 14 (10.2) | 4.3 |
| Extrahepatic metastases only | 34 (25.0) | 10.4 |
| Lungs | 10 (7.4) | 3.1 |
| Brain | 2 (1.4) | 0.6 |
| Bone | 4 (2.9) | 1.2 |
| Lymph nodes | 11 (8.1) | 3.4 |
| Peritoneum | 3 (2.2) | 0.9 |
| Multiple areas | 4 (2.9) | 1.2 |
| Liver and some extrahepatic areas | 19 (13.9) | 5.8 |
| Liver and lungs | 8 (5.8) | 2.8 |
| Liver and pancreas | 2 (1.4) | 0.7 |
| Liver and lymph nodes | 5 (3.7) | 1.7 |
| Liver and bone | 1 (0.7) | 0.3 |
| Liver and others | 3 (2.2) | 1.0 |
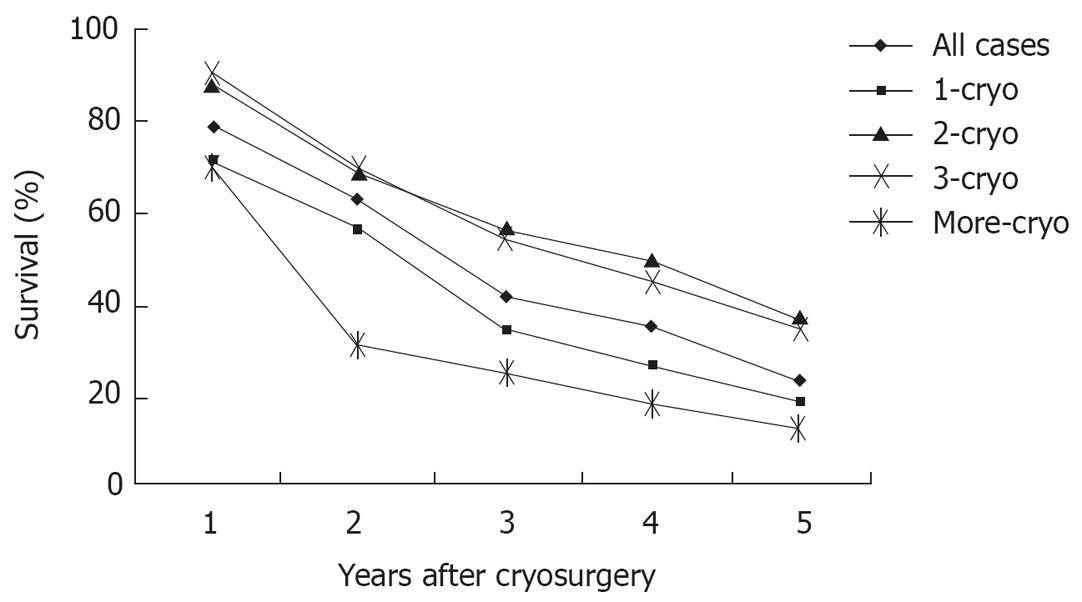
During a median follow-up of 36 mo (7-62 mo), the median survival of all patient was 29 mo (range 3-62 mo). One hundred and ninety six patients (60.1%) died during follow-up, and 130 patients (39.9%) are still alive, with a median survival of 26 and 36 mo, respectively. Overall survival was 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively (Figure 3). Patients with tumor ≤ 3 cm, tumor in right liver lobe, CEA < 100 ng/dL, and post-cryosurgery TACE had a higher survival rate. There was no significant difference in terms of survival based on tumor number, pre-cryosurgery chemotherapy, and timing of the development of metastases (synchronous vs metachronous) (Table 4). Survival was related to the number of cryosurgery procedures performed on the patients. Patients who underwent two or three procedures had an increased survival, compared to those who received cryosurgery only once. However, patients who received cryosurgery on more than three occasions had lower survival (Table 4, Figure 3).
| Median survival (mo) | Survival rate (%) | |||
| 1 yr | 3 yr | 5 yr | ||
| All patients | 29 | 78 | 41 | 23 |
| Tumor size | ||||
| ≤ 3 cmb | 39 | 92 | 64 | 34 |
| > 3 cm | 21 | 70 | 41 | 21 |
| Tumor number | ||||
| < 3 | 38 | 81 | 40 | 20 |
| > 3 | 40 | 78 | 41 | 22 |
| Tumor location | ||||
| Right lobeb | 33 | 87 | 57 | 39 |
| Left lobe | 25 | 72 | 39 | 21 |
| Bilobar | 17 | 65 | 32 | 14 |
| Detection of metastases | ||||
| Synchronous | 30 | 76 | 43 | 24 |
| Metachronous | 29 | 81 | 41 | 21 |
| Metastases detected to cryosurgery | ||||
| < 3 mo | 31 | 74 | 41 | 22 |
| > 3 mo | 29 | 79 | 42 | 23 |
| Pre-cryosurgery chemotherapy | ||||
| Yes | 30 | 75 | 40 | 24 |
| No | 29 | 77 | 41 | 21 |
| Pre-cryosurgery CEA | ||||
| < 100 ng/dLb | 44 | 90 | 57 | 41 |
| > 100 ng/dL | 20 | 73 | 39 | 17 |
| Cryosurgery procedure | ||||
| Once | 21 | 70 | 34 | 19 |
| Twiceb | 38 | 87 | 55 | 36 |
| Thriceb | 39 | 90 | 54 | 34 |
| More | 12 | 69 | 25 | 13 |
| Post-cryosurgery TACE | ||||
| Yesb | 38 | 84 | 57 | 47 |
| No | 18 | 76 | 25 | 15 |
The minor and major adverse effects of cryosurgery are shown in Table 5. A temporary pain in the abdominal right-upper quadrant and fever (about 38°C) were observed in about half the patients. An elevation of serum transaminase levels occurred in 124 patients and normalization was observed within 14 d. Differing degrees of thrombocytopenia were seen in 58 patients, only four of whom received infusion of fresh frozen plasma or platelet concentrate, none had poor consequence. Twenty patients, of whom, 18 had sub-diaphragmatic liver tumor, had right pleural effusion, probably due to the irritative process beneath the diaphragm.
| Adverse effects | No. of patients | % |
| Minor | ||
| Pain | 103 | 31.6 |
| Fever (> 38°C) | 108 | 33.1 |
| Increased liver enzymes | 124 | 38 |
| Thrombocytopenia | 58 | 17.8 |
| Pleural effusion | 20 | 6.1 |
| Major | ||
| Hepatic bleeding | 5 | 1.5 |
| Cryoshock | 1 | 0.3 |
| biliary fistulae | 3 | 0.9 |
| Liver failure | 1 | 0.3 |
| Renal insufficiency | 5 | 1.5 |
| Liver abscess | 3 | 0.9 |
| Acute myocardial infarction and severe arrhythmias | 2 | 0.6 |
There was no cryosurgical mortality. A total of 19 patients developed major adverse effects. Hemorrhage from a cryotreated lesion was seen in five patients, three of whom died of the complication. One patient, who underwent cryosurgery for > 50% of liver volume, died of hepatic failure. One patient, who received cryosurgery for eight large metastases, died of a cryoshock syndrome. Three patients suffered from biliary fistula which resolved with transhepatic drainage. Five patients had temporary renal insufficiency, which presented as increased blood urea nitrogen and creatinine levels for 3-7 d. Two patients developed bacterial hepatic abscess within cryotreated sites and recovered with antibacterial agents and drainage. Two patients, aged 72 and 76 years respectively, died of acute myocardial infarction and severe arrhythmias apparently unrelated to cryosurgery. There were a total of seven patients who died from the main adverse effects after cryosurgery.
Cryosurgery is a treatment in which tumors are frozen and then left in situ to be reabsorbed. Several studies have reported the results of hepatic cryosurgery for treatment of hepatic colorectal metastases[13–16]. Survival after cryosurgery is probably inferior to that achieved by liver resection, but it should be noted that most of the patients undergoing cryosurgery have non-resectable tumors or later stages of the disease. Current long-term follow-up has shown that cryosurgery is an important option for a wide range of non-resectable hepatic colorectal metastases and provides the potential for long-term survival[17].
Until now, cryosurgery for most patients with hepatic colorectal metastases was performed during laparotomy, either as a single modality or in association with liver resection. Operative cryosurgery is still more invasive for the patient. As an advancement of imaging guidance and improvement of cryosurgical apparatus, the percutaneous mode of cryosurgery, a less invasive procedure, has been used for treatment of tumors, and apparently, may be suitable for non-resectable hepatic colorectal metastases.
This study, in which 326 patients with non-resectable hepatic colorectal metastases who underwent percutaneous cryosurgery were followed-up for a median of 36 mo, showed: (1) after cryosurgery, serum CEA in 77.5% of patients with elevated markers returned to normal. (2) After cryosurgery, CR was achieved in 14.6% of patients, PR in 41.1%, and SD in 24.3%. (3) During a median follow-up of 36 mo (7-62 mo), the median survival of all patients was 29 mo (range 3-62 mo). Overall survival was 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively.
Comparing the published results of operative cryosurgery (Table 6), in which the median survival was 21-45 mo, and the 1-, 2-, 3- and 5-years survival was 52%-86%, 36%-62%, 10%-70% and 5%-44%, our results are encouraging, especially in terms of non-resectable tumors.
| Authors | No. of cases | Mode of cryo | Operative mortality (%) | Associated therapy (No. of patients) | Follow-up (mo) | Median survival (mo) | Survival (%) | |||
| 1 yr | 2 yr | 3 yr | 5 yr | |||||||
| Korpan 1997[22] | 63 | OC | 0 | Resection | 6-120 | 60 | 44 | |||
| Wallace 1999[23] | 137 | OC | 0 | Resection | 14 (1-60) | 23 | 86 | 47 | 29 | |
| Weaver 1995[15] | 47 | OC | 4 | Chemo | 26 (24-57) | 62 | ||||
| Ruers 2001[21] | 30 | OC | Resection | 26 (9-73) | 32 | 76 | 61 | |||
| Cha 2001[20] | 21 | OC | Resection | 28 (18-51) | 701 | |||||
| Bilchik 2001[19] | 153 | OC | Chemo | 28 | ||||||
| Goering 2002[18] | 42 | OC | Resection | 45 | 82 | 55 | 39 | |||
| Kerkar 2004[14] | 982 | OC | 54 (9-98) | 33 | 81 | 62 | 48 | 28 | ||
| Jungrai Thmayr 2005 | 17 | OC | 0 | 23 (2-65) | 21 | 52 | 36 | 10 | 5 | |
| This study 2007 | 326 | PC | 0 | TACE | 32 (7-62) | 29 | 78 | 62 | 41 | 23 |
Similar to operative cryosurgery, the main problem in the face of percutaneous cryosurgery is recurrence. In our patients, 47.2% had recurrence during a median follow-up of 32 mo (range, 7-61). The liver was the main site of recurrence, 61% of which occurred in the liver only, and 13.9% in the liver and some extrahepatic locations. Extrahepatic recurrence was mainly seen in the lungs and lymph nodes. The overall recurrence rate was lower than the 44% in a mean follow-up of 16 mo, reported by Adam[13], and much lower than the 78% reported by Weaver[16]. It is important to point out that in our patients, the recurrence rate at cryotreated site, including at cryotreated site only as well as both of cryotreated site and the remaining areas of liver, was only 15.3% for patients with recurrence and 6.4% for all cases, which is significantly lower than the 58.8% reported by Jungraithmayr[24]. Obviously, the decreased tumor recurrence is related to better survival.
This study showed patients with lesions ≤ 3 cm had an increased survival rate compared with those with lesions > 3 cm, with a median survival of 39 and 21 mo, respectively. This may have been due to the larger tumor in the vicinity of large vessels and exposure to the heat sink effect. The warming effect of blood flow can cause insufficient cryodestruction of the tumor. Pearson et al[25] have reported that 66.7% of local recurrence occurs directly in the vicinity of the vena cava or a large vessel. No significant correlation has been found between the number of metastases and survival. Patients with tumor in the right hepatic lobe have higher survival compared with those with left lobe or bilateral tumors, which may be because the latter location is closer to large vessels.
This study also appeared to show a correlation between poor survival and CEA level > 100 ng/dL, with a median survival of 18 mo, which is much less than the 38 mo median survival for patients with lower CEA (P < 0.01). The result is consistent with that reported by Weaver et al[1516] who showed that patients with CEA > 100 ng/dL prior to cryosurgery had only 10 mo median survival, while the median survival of patients with CEA lower than that level was 17-19 mo. The poor outcome of patients with higher CEA may be related to the biological behavior of CEA-secreting tumors.
According to the results of this study, the possibility of repeat percutaneous cryosurgery may be a factor which brings about better survival and low recurrence. Patients who received two or three cryosurgery procedures had longer survival. In contrast to operative cryosurgery, percutaneous cryosurgery may be performed many times because of its convenience and low intervention. As a result, the recurrence in liver and extrahepatic metastases may be conveniently treated. In this series, there were 12 and 6 patients, respectively, with lung and pancreas metastases, who were treated by percutaneous cryosurgery.
As shown in this study, patients who received post-cryosurgery TACE had the higher survival compared to patients who did not receive TACE (5-year survival of 47% vs 15%). Post-resection TACE has been shown to decrease the recurrence rate for patients with HCC[26]. The effect, therefore, may be an additional factor for the longer survival in our patients. We have used combination of percutaneous cryosurgery and absolute ethanol injection for treatment of HCC with good results[27]. The strategy may be suitable for hepatic colorectal metastases.
In this study, a total of 526 procedures of cryosurgery in all patients were safely performed percutaneously. There were 151 patients who underwent repeat cryosurgery, as many as two to four procedures, for recurrent tumors in the liver and extrahepatic loci. There was no treatment-related mortality. Although about one third of patients had adverse effects, such as pain, fever, increased liver enzymes, thrombocytopenia and pleural effusion, they were generally self-limited without poor outcome. Cryoshock, as the most serious complication of hepatic cryosurgery, was observed in one of our patients, with an incidence of 0.3%, which is lower than the 1% in patients who underwent operative hepatic cryosurgery, based on a worldwide survey[28]. Other major adverse effects, such as hepatic bleeding, biliary fistula, liver failure, renal insufficiency and liver abscess, were observed in 0.3%-1.5% of our patients; however, the incidence is no higher than that in patients undergoing operative hepatic cryosurgery[2930]. Therefore, percutaneous cryosurgery is a safe technique for liver surgery.
In conclusion, the results of this study clearly show that percutaneous cryosurgery is a safe modality for hepatic colorectal metastases. Rather than an alternative to resection, the technique should be regarded as a complement to hepatectomy and as an additional means to achieve tumor eradication when total excision can not be accomplished.
Nearly 50% of patients who have colorectal carcinoma will develop liver metastases, which are a frequent cause of death. Without treatment, the average survival of patients with multiple lesions is 5-10 mo, and the 5 years survival rate is 1%. Liver resection is the only established, potentially curative treatment for patients with hepatic colorectal metastases confined to the liver, however, most patients have non-resectable tumors, even when they have no extrahepatic disease. If patients have more than three lesions or have anatomical constraints at presentation, only 5%-10% are suitable for liver resection. Palliative resection is of no significant benefit. Chemotherapy, including intra-arterial continuous infusion, is the most commonly used therapeutic modality but prolongation of survival has not been proven. Irradiation of liver metastases has been shown to relieve pain, but median survival does not appear to be prolonged. Therefore, the search for new treatment modalities for patients with non-resectable hepatic colorectal metastases is very urgent.
Cryosurgery has increasingly been recognized as a safe and effective alternative treatment modality that is used alone or in conjunction with hepatic resection for a selected group of patients with non-resectable disease confined to the liver. It has shown some promising results, with a median survival > 2 years in most published studies and the prospect of long-term disease-free survival or cure for some patients.
Cryosurgery in most patients with hepatic colorectal metastases has been performed with open laparotomy. With improved imaging techniques and cryosurgical equipment, percutaneous cryosurgery, as a minimally invasive alternative for open procedure, is increasingly used for treatment of liver tumors. This study of 326 patients with hepatic colorectal metastases who underwent percutaneous cryosurgery demonstrates encouraging efficacy, with overall survival of 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively. These survival rates are much greater than those achieved using other treatments in patients with non-resectable liver metastases from colorectal carcinoma. Moreover, the incidence of complications is no higher than that in patients who underwent operative hepatic cryosurgery.
Percutaneous cryosurgery is a safe and feasible approach for treatment of patients with non-resectable hepatic colorectal metastases. Rather than an alternative to resection, it is to be regarded as a complement to hepatectomy and as an additional means to achieve tumor eradication when total excision can not be accomplished.
Ablation destroys tumors locally without requiring resection, with effectiveness comparable to resection. Cryosurgery is one of the ablation techniques. Cryosurgery is referred to as cryotherapy or cryoablation, and is the in situ destruction of undesirable tissue by localized freezing. It has become an effective technique for treating tumors of solid organs such as liver, prostate, kidneys, lungs, and has advantages over other surgical techniques. Percutaneous cryosurgery is a minimally invasive technique, which is usually performed by the insertion of one or more cryosurgical probes into the tumor under guidance of ultrasound, CT or MRI.
This is a valuable paper, which describes the efficacy and safety profile of percutaneous cryosurgery. A prospective trial that compares surgical cryoablation and other forms of ablation in terms of patient survival is necessary to confirm the effects of ablation for non-resectable liver cancer.
| 1. | Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246. |
| 2. | Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278-284. |
| 3. | Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946. |
| 4. | Moug SJ, Horgan PG. The role of synchronous procedures in the treatment of colorectal liver metastases. Surg Oncol. 2007;16:53-58. |
| 5. | Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718-726. |
| 6. | Khatri VP, Chee KG, Petrelli NJ. Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol. 2007;16:71-83. |
| 7. | Alberts SR. Evolving role of chemotherapy in resected liver metastases. J Clin Oncol. 2006;24:4952-4953. |
| 8. | Onik G, Rubinsky B, Zemel R, Weaver L, Diamond D, Cobb C, Porterfield B. Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer. 1991;67:901-907. |
| 9. | Tandan VR, Harmantas A, Gallinger S. Long-term survival after hepatic cryosurgery versus surgical resection for metastatic colorectal carcinoma: a critical review of the literature. Can J Surg. 1997;40:175-181. |
| 10. | Onik GM, Atkinson D, Zemel R, Weaver ML. Cryosurgery of liver cancer. Semin Surg Oncol. 1993;9:309-317. |
| 11. | Ravikumar TS, Kane R, Cady B, Jenkins R, Clouse M, Steele G Jr. A 5-year study of cryosurgery in the treatment of liver tumors. Arch Surg. 1991;126:1520-1523; discussion 1523-1524. |
| 12. | Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1-3. |
| 13. | Adam R, Akpinar E, Johann M, Kunstlinger F, Majno P, Bismuth H. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg. 1997;225:39-48; discussion 48-50. |
| 14. | Kerkar S, Carlin AM, Sohn RL, Steffes C, Tyburski J, Littrup P, Weaver D. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery. 2004;136:770-779. |
| 15. | Weaver ML, Atkinson D, Zemel R. Hepatic cryosurgery in treating colorectal metastases. Cancer. 1995;76:210-214. |
| 16. | Weaver ML, Ashton JG, Zemel R. Treatment of colorectal liver metastases by cryotherapy. Semin Surg Oncol. 1998;14:163-170. |
| 17. | Yan DB, Clingan P, Morris DL. Hepatic cryotherapy and regional chemotherapy with or without resection for liver metastases from colorectal carcinoma: How many are too many? Cancer. 2003;98:320-330. |
| 18. | Goering JD, Mahvi DM, Niederhuber JE, Chicks D, Rikkers LF. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg. 2002;183:384-389. |
| 19. | Bilchik AJ, Wood TF, Allegra D, Tsioulias GJ, Chung M, Rose DM, Ramming KP, Morton DL. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657-662; discussion 662-664. |
| 20. | Cha C, Lee FT Jr, Rikkers LF, Niederhuber JE, Nguyen BT, Mahvi DM. Rationale for the combination of cryoablation with surgical resection of hepatic tumors. J Gastrointest Surg. 2001;5:206-213. |
| 21. | Ruers TJ, Joosten J, Jager GJ, Wobbes T. Long-term results of treating hepatic colorectal metastases with cryosurgery. Br J Surg. 2001;88:844-849. |
| 22. | Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg. 1997;225:193-201. |
| 23. | Wallace JR, Christians KK, Pitt HA, Quebbeman EJ. Cryotherapy extends the indications for treatment of colorectal liver metastases. Surgery. 1999;126:766-772; discussion 772-774. |
| 24. | Jungraithmayr W, Burger D, Olschewski M, Eggstein S. Cryoablation of malignant liver tumors: results of a single center study. Hepatobiliary Pancreat Dis Int. 2005;4:554-560. |
| 25. | Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, Granchi J, Curley SA. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592-599. |
| 26. | Li JQ. The modalities for decreasing post-resection recurrence of liver cancer. Zhonghua Ganzangbing Zazhi. 2000;8:77. |
| 27. | Xu KC, Niu LZ, He WB, Guo ZQ, Hu YZ, Zuo JS. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol. 2003;9:2686-2689. |
| 28. | Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-113; discussion 113-114. |
| 29. | Sarantou T, Bilchik A, Ramming KP. Complications of hepatic cryosurgery. Semin Surg Oncol. 1998;14:156-162. |
| 30. | Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205:342-356. |