INTRODUCTION
The Gram negative bacterium H pylori is involved in the pathogenesis of several gastrointestinal diseases, ultimately leading to gastric carcinoma[12]. In the gastric mucosa, the majority of the bacteria is found within the mucus layer, but can be also attached to gastric epithelial cells[3], a crucial step for the maintenance, spreading and severity of the infection. This attachment is mediated by the interaction of bacterial molecules, such as adhesins and LPS[4], with gastric cell surface ligands such as glycolipids and glycoproteins. MUC1 is a membrane glycoprotein that protects epithelial surfaces and has been recently identified as an H pylori binding target[56]. Extracellular MUC1 variable number of tandem repeats (VNTR) domain is highly glycosylated[7], presenting carbohydrate structures (e.g. Lewis b carbohydrate antigen) involved in the binding of H pylori through its adhesins BabA and SabA[89]. Furthermore this repetitive region shows extensive allelic variation ranging from 25-125 repeat units[12]. The relevance of MUC1 VNTR variability for H pylori adhesion to gastric cells remains to be clarified.
In this work we tested the hypothesis that MUC1 VNTR polymorphism affects the H pylori adhesion to gastric cells and thus plays an important role in the colonization of gastric mucosa. We used H pylori strains with different pathogenicity (strain HP26695 and strain HPTx30a) co-cultured with gastric cell lines GP202 and MKN45, and GP202 clones expressing recombinant MUC1 with different VNTR lengths. Adhesion was evaluated by an enzyme linked immunosorbent assay (ELISA)-based adhesion assay.
The results showed that MUC1 VNTR polymorphism influences the binding of H pylori to gastric cells. Furthermore, higher adhesion was observed in co-cultures with the pathogenic strain (HP26695) when compared to the non-pathogenic strain (HPTx30a) and GP202 cell line when compared to the MKN45 cell line. This work contributes to the understanding of the interplay between host and bacterial factors in H pylori infection pathogenesis.
MATERIALS AND METHODS
Cell lines
We used two gastric carcinoma cell lines: GP202, previously established in our laboratory[13] from a signet ring cell gastric carcinoma that constitutively expresses MUC1 and MKN45 (Japan Health Sciences Foundation).
GP202 clones expressing recombinant MUC1 with different VNTR lengths[14] were previously established by stable transfection with an eukaryotic expression vector pHb-APr1-neo containing subcloned epitope-tagged MUC1 (FLAG-MUC1) cDNAs with different number of TR units (0, 3, 9 and 42 repeats, respectively GP202-dTR, GP202-3TR, GP202-9TR and GP202-42TR)[15]. GP202-Neo was obtained by transfection with empty vector.
The parental cell lines and transfectants were cultured in 150 cm2 flasks at 37°C in a humidified 5% CO2 incubator and maintained in RPMI 1640 medium (with Glutamax and 25 mmol/L Hepes) supplemented with 10% fetal bovine serum and 50 &mgr;g/mL gentamicin. Media was changed every 3 d to 4 d, and the cells were passaged when they reached 80% to 90% confluence using 0.05% trypsin-0.53 mmol/L ethylenediamine tetra-acetic acid in Hank’s balanced salt solution. Cell culture reagents were obtained from Invitrogen (Carslbad, CA, USA).
H pylori strains
Two H pylori strains were used in this study: the pathogenic strain HP26695 (vacA s1/m1, cag PAI+, ATCC 700392) and the non-pathogenic strain HPTx30a (vacA s2/m2, cag PAI-, ATCC 51932). Bacteria were grown on Trypticase soy agar with 5% sheep blood (BioMérieux) at 37°C in microaerobic conditions.
ELISA assay
Quantitative evaluation of H pylori adhesion to gastric cells was performed by ELISA, as previously described[16], with some modifications. Briefly, cells were cultured in 96 well plates and allowed to form confluent monolayers. Cells were washed and H pylori suspension was added in a 200:1 bacteria to cell ratio (MOI) and incubated for 60 min. Cells were washed and fixed at 4°C with 8% paraformaldehyde for 60 min. Endogenous peroxidase was inactivated by addition of 1% H2O2 in methanol. After washing with PBS, anti-H pylori monoclonal antibody MAB922 (Chemicon, USA) was added overnight, 4°C, followed by addition of peroxidase-conjugated goat anti-mouse immunoglobulins (Santa Cruz Biotechnology) 30 min, RT. Tetramethylbenzidine (TMB) (Sigma, USA) was added and reaction stopped with 1 mol/L HCl. Plates were read in a 680 ELISA microplate reader (Bio-Rad, USA) at 450 nm. OD values were used as the index of the number of H pylori adhering to cells[16]. Two sets of triplicates were made for each assay.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney test, StatView Software version 5.0 (SAS Institute). A P value of less than 0.05 was accepted as statistically significant.
RESULTS
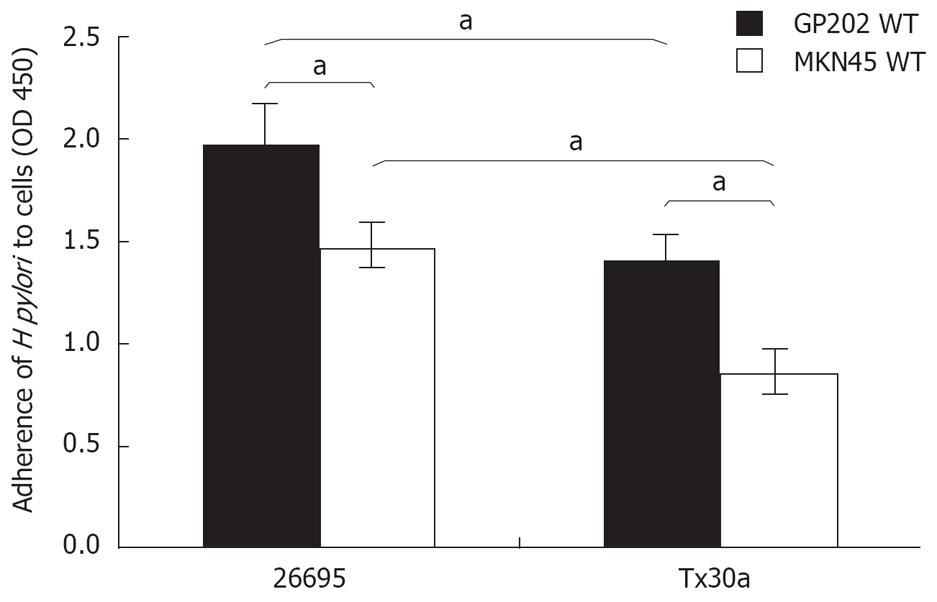
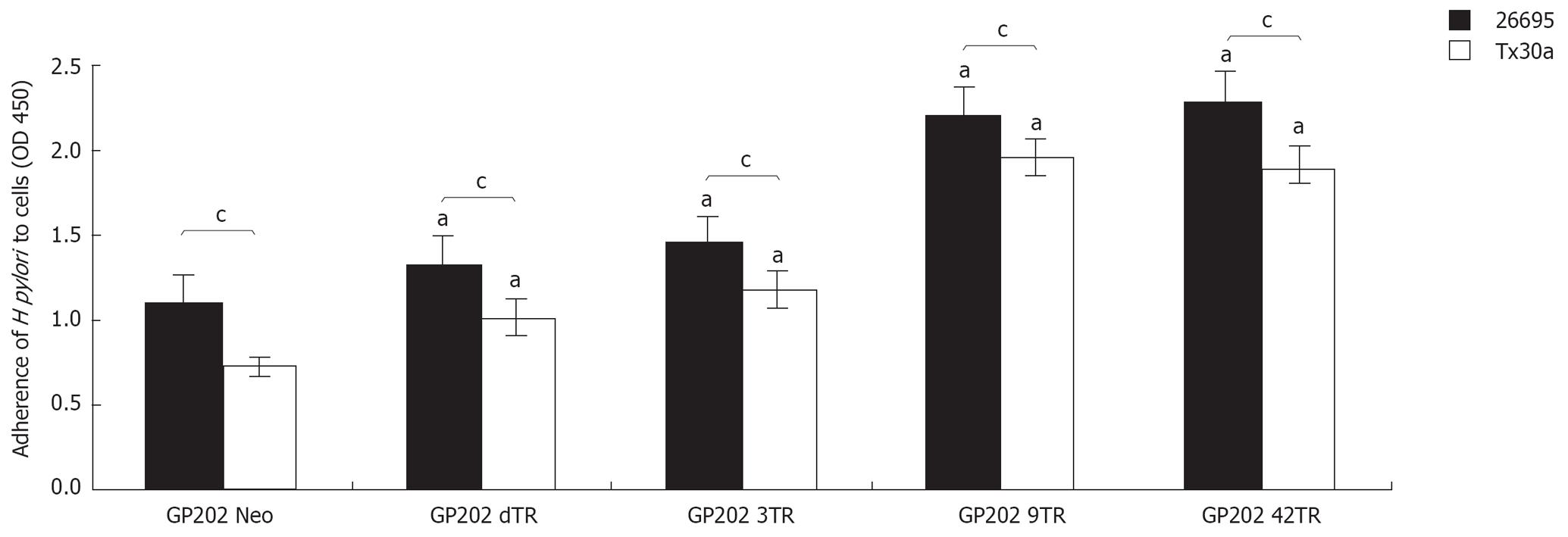
Evaluation of H pylori adhesion shows that pathogenic strain HP26695 has significantly (P < 0.05) higher adhesion values for both GP202 and MKN45 cell lines (1.97 ± 0.10 and 1.47 ± 0.06) when compared with the non-pathogenic strain HPTx30a (1.40 ± 0.15 and 0.85 ± 0.15; Figure 1). This statistically significant association between pathogenicity and higher adhesion (strain HP26695 vs HPTx30a) is also observed for the GP202 MUC1 recombinant clones (GP202-Neo 1.1 ± 0.10 vs 0.72 ± 0.06; GP202-dTR 1.32 ± 0.09 vs 1.0 ± 0.10; GP202-3TR 1.45 ± 0.08 vs 1.18 ± 0.05; GP202-9TR 2.2 ± 0.12 vs 1.96 ± 0.12; and GP202-42TR 2.3 ± 0.07 vs 1.89 ± 0.11; Figure 2). Furthermore, GP202 cell line shows higher adhesion levels than MKN45 cell line for both bacteria strains (HP26695 strain 1.97 ± 0.10 vs 1.47 ± 0.06; HPTx30a strain 1.40 ± 0.15 and 0.85 ± 0.15; Figure 1).
Figure 1 Adhesion of HP26695 and HPTx30a H pylori strains to GP202 and MKN45 gastric cell lines.
aP < 0.05.
Figure 2 Adhesion of HP26695 and HPTx30a H pylori strains to GP202 transfectants GP202-Neo, GP202-dTR, GP202-3TR, GP202-9TR and GP202-42TR.
aP < 0.05, compared to the control (GP202 Neo) and cP < 0.05.
Adhesion of both H pylori strains (HP26695 and HPTx30a) is significantly higher in all the GP202-MUC1 transfectants over-expressing MUC1 (GP202-dTR 1.32 ± 0.09 and 1.0 ± 0.10; GP202-3TR 1.45 ± 0.08 and 1.18 ± 0.05; GP202-9TR 2.2 ± 0.12 and 1.96 ± 0.12; GP202-42TR 2.3 ± 0.07 and 1.89 ± 0.11) when compared with the control, GP202 Neo (1.1 ± 0.10 and 0.72 ± 0.06, Figure 2). There is also an association between the increased number of Tandem Repeats (GP202-9TR and GP202-42TR) and the increased adhesion, for both strains (Figure 2).
DISCUSSION
Epidemiological studies and animal models have shown that H pylori chronic infection is associated with several gastric pathologies, ranging from asymptomatic gastritis to gastric adenocarcinoma and MALT lymphoma[12]. The different consequences of the infection suggest that several factors from the host and the bacteria are involved in the bacteria-host interactions, being the pathogenic potential dependent upon the molecular context of the colonization of gastric mucosa. To date several factors involved in the H pylori infection have already been identified (e.g. bacterial adhesins, host mucins and pro-inflammatory cytokines) however the complete mechanism remains to be clarified[17–19].
Adhesion of H pylori to gastric mucosa is a fundamental step for epithelium colonization. Different adhesion mechanisms, commonly targeting carbohydrate structures present on gastric cells surface, have been identified[4] with H pylori ligands including, among others, blood group antigens on mucins and glycolipids[8–1120–26].
The best-characterized H pylori adhesin is BabA, that mediates a strong adhesion between the bacteria and Leb blood group antigen expressed on the surface of epithelial cells[827]. This work showed that adhesion is a relevant feature of H pylori pathogenicity potential, with significantly higher adhesion levels observed for the HP26695 (pathogenic strain) when compared to the HPTx30a (non-pathogenic strain) in both cell lines. Considering that both strains don’t express BabA adhesin[28], the observed differences can not be explained through the BabA binding model, what suggests that other bacterial molecules are involved in the adhesion process.
Another important observation is that there is a higher adhesion of HP26695 and HPTx30a strains to GP202 cell line when compared with MKN45 cell line. This reflects different expression levels and availability of ligands at the cells surface. Previous characterization of mucins and carbohydrate expression on GP202 and MKN45 cell lines showed that Leb has a significantly higher expression in GP202 cell line[29]. Still, this difference might not be relevant since BabA is not present in both bacterial strains[28]. In addition, the MUC1 expression is identical for both cell lines[29] and therefore can not be held responsible for the observed differences. GP202 has a higher expression of other carbohydrate antigens (Lea and Ley)[2930] compared to MKN45, that might be involved in H pylori binding interactions. Moreover, additional ligands/interactions that are not yet explored may also exist that can explain this difference in adhesion levels between cell lines.
In order to study the influence of MUC1 VNTR variability in H pylori binding, we used GP202, the cell line that showed higher bacteria adhesion and we analyzed GP202 transfected clones expressing recombinant MUC1 with a different number of repeats. These clones overexpress similar levels of recombinant MUC1[14]. We observed that MUC1 VNTR polymorphism has influence in the extent of H pylori binding to gastric cells, with the higher adhesion levels observed in clones with larger VNTR regions. This may be due to the fact that MUC1 with larger Tandem Repeat regions contains more potential glycan receptors, thus potentially providing more bacterial binding sites. Moreover, we have previously shown that differences in VNTR length lead to glycosylation changes in the MUC1 Tandem Repeat[14], which may also contribute to the altered adhesion observed. Detailed evaluation of the results showed a small increase between the adhesion of GP202-NEO (control) and GP202-dTR that may be explained by the overexpression of MUC1 in recombinant clone GP202-dTR[14] and by the potential presence of O-glycosylated binding sites outside the VNTR region. No significant difference was observed between the adhesion of the bacteria to GP202-9TR and to GP202-42TR clones. We have previously observed the overexpression of MUC1 underglycosylated forms in GP202-42TR[14], which might explain why the adhesion levels are not proportional to VNTR size.
All these observations are important for understanding the bacterial and host molecular context of the colonization of gastric mucosa. Identification of a pathogenesis background, based upon host susceptibility traits like MUC1 VNTR polymorphism, will help to identify candidates more prone to bacterial colonization and patients more resilient to eradication strategies.
COMMENTS
Background
More than half of the world population is persistently infected by H pylori. Adhesion of the bacteria to the gastric mucosa is essential for attachment and infection. Therefore it is important to know host and bacterial factors that condition the adhesion.
Innovations and breakthroughs
The study of host factors that influence the binding of H pylori to gastric cells may help to identify candidates more prone to bacterial colonization and patients more resilient to eradication strategies.
Applications
These findings may help to develop screening methods to identify candidates more prone to bacterial colonization and to develop more efficient eradication strategies, as well as to develop strategies to prevent or minimize H pylori binding to the gastric mucosa.
Peer review
This is a good study designed to elucidate that MUC1 VNTR polymorphism affects H pylori adhesion to gastric cells. The results are informative and potentially helpful for prevention of H pylori binding to the gastric mucosa.