Published online Mar 7, 2008. doi: 10.3748/wjg.14.1313
Revised: January 29, 2008
Published online: March 7, 2008
Good preparation before endoscopic procedures is essential for successful visualization. The small bowel is difficult to evaluate because of its length and complex configuration. A meta-analysis was conducted of studies comparing small bowel visualization by capsule endoscopy with and without preparation. Medical data bases were searched for all studies investigating the preparation for capsule endoscopy of the small bowel up to July 31, 2007. Studies that scored bowel cleanness and measured gastric and small bowel transit time and rate of cecum visualization were included. The primary endpoint was the quality of bowel visualization. The secondary endpoints were transit times and proportion of examinations that demonstrated the cecum, with and without preparation. Meta-analysis was performed with StatDirect Statistical software, version 2.6.1 (http://statsdirect.com). Eight studies met the inclusion criteria. Bowel visualization was scored as “good” in 78% of the examinations performed with preparation and 49% performed without (P < 0.0001). There were no significant differences in transit times or in the proportion of examinations that demonstrated the cecum with and without preparation. Capsule endoscopy preparation improves the quality of small bowel visualization, but has no effect on transit times, or demonstration of the cecum.
- Citation: Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: A meta-analysis. World J Gastroenterol 2008; 14(9): 1313-1317
- URL: https://www.wjgnet.com/1007-9327/full/v14/i9/1313.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1313
Good preparation before endoscopic procedures is essential for successful visualization. It is also an important factor for patient safety, quality of care, and cost efficiency[12]. The small bowel is difficult to evaluate because of its length and complex configuration. The recently developed Given Diagnostic Imaging System (PillCam, Given Imaging Ltd., Yoqneam, Israel) for evaluation of pathologies of the small bowel[3–7] can identify lesions that cannot be detected by other techniques[89]. The preparation recommended by the manufacturer is a 12-hour fast after 24-hour intake of clear liquids.
Studies of the effect of bowel preparation for capsule endoscopy on optimal visualization, gastric and small bowel transit times, and rate of cecum demonstration have sometimes reached different conclusions[10–17]. Sood et al[18], in MRI studies, found no significant difference in transit time between patients prepared with polyethylene glycol or standard methods. In our previous studies, the use of sodium phosphate preparation was not associated with a significant change over fasting in gastric or small bowel transit times, although it yielded a significantly better view of the mucosa[1011]. Others reported that both polyethylene glycol and sodium phosphate have a marked accelerating effect on small intestinal transit time[1920].
In the present study, we performed a meta-analysis of studies comparing preparation with no preparation before capsule endoscopy of the small bowel. The primary endpoint was the quality of small bowel visualization. The secondary endpoints were gastric and small bowel transit times and proportion of examinations demonstrating the cecum.
The primary question was: What is the contribution of bowel preparation to successful capsule endoscopy examination? This raised the subsidiary question of a possible difference in the rates of excellent and good cleaning of the small bowel with and without preparation. In addition, we sought to determine the impact of preparation on gastric transit time, small bowel transit time, and arrival of the capsule to the cecum with the battery still functioning.
Our study was based on controlled studies of the quality of small bowel capsule examination with and without prior preparation. Two studies of our group were included in the meta-analysis[1011]. There was no overlap between the patient populations in these two studies.
We conducted a bibliographic search of the PubMed (MEDLINE), EMBASE, COCHRANE LIBRARY (Cochrane Database of Systemic Reviews and the Cochrane Controlled Trial Register), CINAHL, and AMED databases up to July 31, 2007 using the following keywords: “capsule endoscopy”, “M2A” (mouth-to-anus), and “PillCam”. The search was directed at English-language medical journals. All papers identified by the electronic database search were examined and additional references were identified from the references listed in each paper.
Case-control studies investigating the preparation for capsule endoscopy of the small bowel which employed a scoring system for bowel cleanness and measured gastric and small bowel transit time and rate of cecum demonstration were included.
Studies that focused on esophageal or colonic examination, did not directly compare preparations, had no control, or did not measure transit times were excluded from the analysis.
For each study, the following variables were extracted and entered into an Excel data sheet: author, journal, year of publication, number of participants, type of preparations, proportion of good bowel visualization, gastric transit time, small bowel transit time, and proportion of cecum demonstration. The meta-analysis was performed with the StatDirect Statistical software, version 2.6.1 (http://statsdirect.com). Heterogeneity was checked using χ2 test (Q-statistics) with significance of P < 0.05. With the assumption that the studies are a random sample from a population of studies, we choose to use a random effects model. Forest plots were constructed for visual presentation of the individual studies proportions and the pooled proportion[21].
Our search yielded 550 studies of capsule endoscopy, of which 8 investigated the preparation for small bowel capsule endoscopy and otherwise met the inclusion criteria[10–17].
Five out of these studies compared the proportion of “good” scores between the groups[10–1317]. The findings are shown in Table 1. A total of 237 patients were included, 130 with and 107 without preparation.
Seven out of these studies included a comparison of gastric and small bowel transit times and in proportion to examinations of cecum demonstration[101113–17]. The findings are shown in Table 2. A total of 401 patients were included, 221 with and 180 without preparation.
| Author | Ref. | Prep. | No. with | No. without | GTT with (min) | GTT without (min) | SBTT with (min) | SBTT without (min) | Cecum reached with | Cecum reached without |
| Niv Y, 2004 | 10 | Na-P | 22 | 10 | 25.0 | 18.0 | 300 | 333 | 16 | 5 |
| Viazis N, 20042 | 17 | PEG 2L | 40 | 40 | 36.2 | 44.1 | 291.8 | 304.6 | 32 | 26 |
| Niv Y, 2005 | 11 | Na-P | 23 | 23 | 25.0 | 40.0 | 341 | 241 | 18 | 18 |
| Ben-Soussan E, 2005 | 13 | PEG 2L | 26 | 16 | 45.7 | 25.5 | 288 | 271 | 24 | 16 |
| Fireman Z, 2005 | 14 | PEG 1L or Na-Por Erythro | 551 | 401 | 39.7 | 45.5 | 228 | 218 | NM | NM |
| Dai N, 2005 | 16 | PEG 4L | 33 | 28 | 13.0 | 14.0 | 213 | 253 | 32 | 22 |
| Caddy GR, 2006 | 15 | Erythro | 22 | 23 | 50.5 | 38.4 | 304.4 | 302.6 | 7 | 5 |
| Total | 166 | 140 | 129 | 92 | ||||||
| Average ± SD | 33.5 ± 13.2 | 32.2 ± 12.8 | 280.8 ± 44.9 | 274.7 ± 40.6 | ||||||
| Proportion | 0.76 | 0.68 | ||||||||
| P | 0.296 | 0.155 | 0.38 |
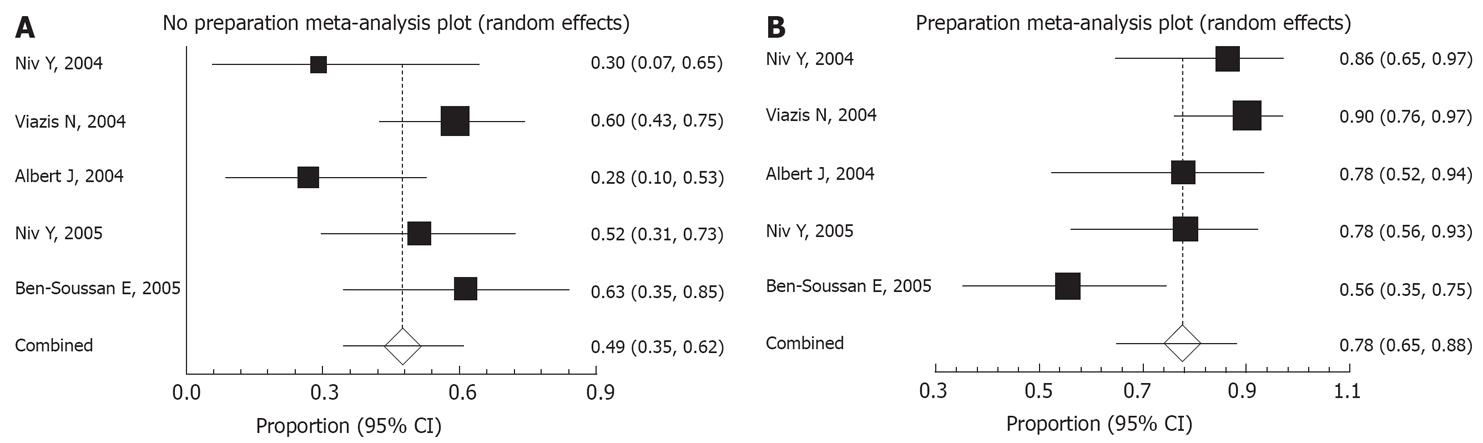
A “good” score was similarly defined in the five papers found suitable for comparison of preparations. In our two papers[1011] we used a score of 3 grades, where “good” was defined as a good mucosal visualization in more than 80% of the small bowel transit time. Albert and coworkers[12] used a score of 4 grades, and we used their scores 0 and 1 as “good”, when there was no limitation for interpretation. Viazis and coworkers used the term adequate when more than 90% of the small bowel transit time and the mucosa was clear[17], and Ben-Soussan and colleagues, using a score of 4 grades, defined “good or excellent” when visibility was good in more than 75% of the small bowel transit time[13]. A “good” score was documented for 78% of the patients with preparation (95% CI, 65%-88%) compared to 49% of the patients without preparation (95% CI, 35%-62%; P < 0.0001; Figure 1).
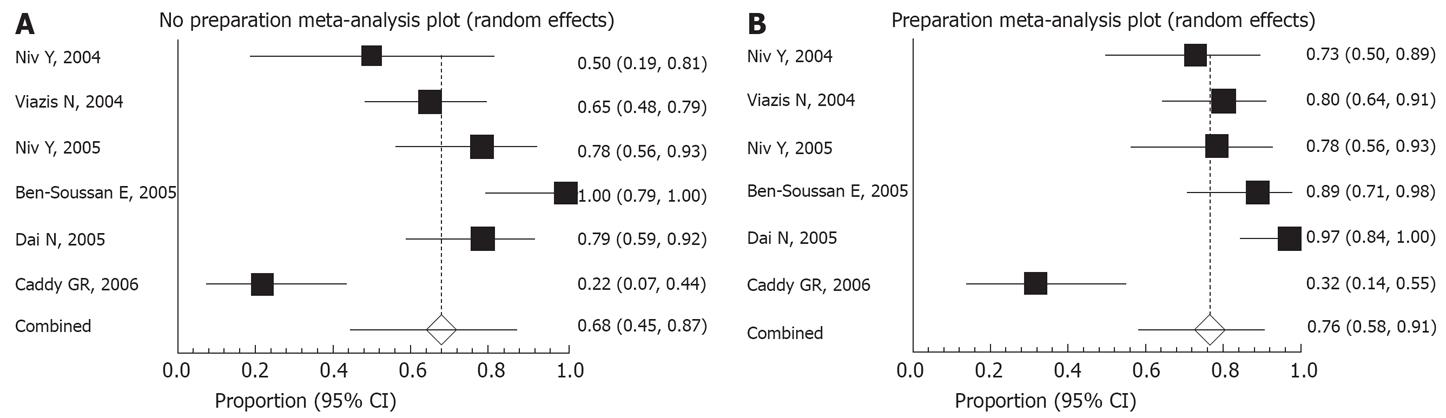
The capsule reached and visualized the cecum in 76% of the patients with preparation and 68% without. This difference did not reach statistical significance (Figure 2).
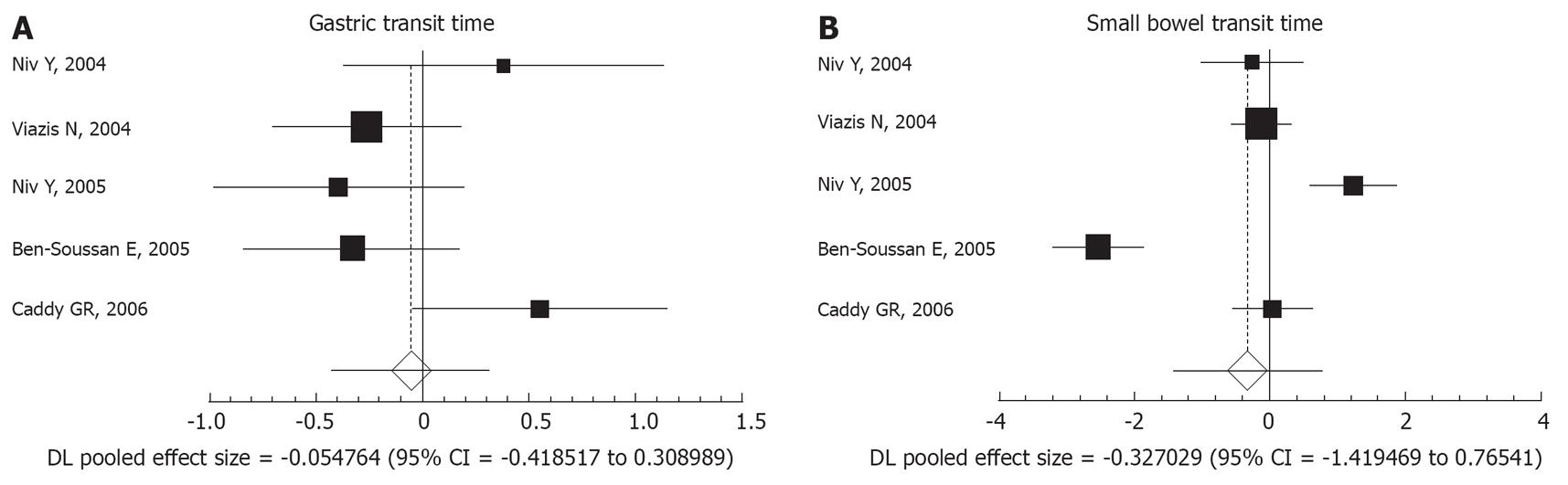
There was no statistically significant difference between the endoscopies performed with or without preparation in gastric transit time (pooled effect size, -0.054; 95% CI, -0.418 to 0.308) or small bowel transit time (pooled effect size, -0.327; 95% CI, -1.419 to -0.765; Figure 3A and B).
Capsule endoscopy is a new technology, and there is still no consensus regarding the proper preparation. The manufacturer recommends only a 12-h fast after 24-h intake of clear liquids. It may be in the interest of the manufacturer to recommend such a simple preparation, so that the procedure will be easy to perform. However, since the procedure is costly, time consuming, and not usually repeated, it is critical to optimize the quality of visualization, which may be impaired by secretions, bubbles or coating of the capsule with intestinal residue.
Few studies have compared different preparation strategies, using different approaches, designs, and methods. Two controlled studies by our group found that the sodium phosphate method provided better visualization than the standard method, with significantly less and later-appearing turbid fluid in the small bowel lumen, which could block the visual field of the capsule[1011]. Our results were in agreement with the prospective, randomized, controlled trial of Viazis et al[17], which demonstrated a significant advantage of preparation with polyethylene glycol over fasting alone. The findings in our control group were also similar to the control findings of Viazis et al[17] in terms of transit times, percentage of procedures in which the cecum was reached, and diagnostic yield (secondary endpoint). Taken together, the data from our nonrandomized comparison case-control studies[1011] and the randomized study of Viazis et al[17] indicate that bowel preparation with either sodium phosphate or polyethylene glycol is superior to simple fasting for capsule endoscopy. Albert and colleagues used simethicone for bubble absorption and improved the visibility of the small bowel mucosa[12]. Thus their paper was added to this meta-analysis even though simethicone is not a classic preparation drug and may not be considered equivalent to polyethylene glycol or to sodium phosphate[12].
Only 3 papers looked at the diagnostic yields as a secondary endpoint, since it would be much more useful to compare more definite outcomes such as number and type of lesions visualized in attempting to compare the effect of preparation and no preparation[111317]. Ben-Soussan and colleagues found no association between preparation and diagnostic yield of the capsule examination[13], while we and Viazis found a significant advantage of preparation for findings more lesions[1117]. Thus, this point is still controversial.
The main weakness of the study is in the different preparation regimens, and different scores used by the individual centers, sometimes addressed to evaluate different aspects of the small bowel cleansing: amount of intraluminal fluid, gas or air bubbles, and percentage of “free” mucosal surface.
Our search yielded 8 studies comparing preparation with sodium phosphate, polyethylene glycol or simethicone in one group of patients with simple fasting in another group, before capsule endoscopy of the small bowel. The results of the meta-analysis clearly demonstrated that although preparation improves the quality of small bowel visualization, it has no significant effect on gastric transit time, small bowel transit time, or cecum demonstration. The main limitation of our meta-analysis is the diversity of the methods used by the different trials. All the studies were controlled, but only one of them was randomized[17]. Two studies compared different strategies used by different centers, and 5 studies compared 2 different periods with changed strategies. Nonetheless, the study groups in every study were very similar in their demographic and clinical data. Most of the patients were referred for the capsule examination to evaluate occult gastrointestinal bleeding. Co-morbidity was also similar between the groups, and no significant alterations in transit times were anticipated. Thus, further studies are needed to definitively establish the association among motility factors, battery life-time, good visualization of the bowel, and completeness of the examination.
| 1. | Abuksis G, Mor M, Segal N, Shemesh I, Morad I, Plaut S, Weiss E, Sulkes J, Fraser G, Niv Y. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol. 2001;96:1786-1790. |
| 2. | Abuksis G, Niv Y. Predictors of inadequate colonic preparation for colonoscopy. Am J Gastroenterol. 2002;97:216. |
| 3. | Lewis B, Goldfarb N. Review article: The advent of capsule endoscopy--a not-so-futuristic approach to obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2003;17:1085-1096. |
| 5. | Herrerias JM, Caunedo A, Rodriguez-Tellez M, Pellicer F, Herrerias JM Jr. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy. 2003;35:564-568. |
| 6. | Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576-584. |
| 7. | Liangpunsakul S, Chadalawada V, Rex DK, Maglinte D, Lappas J. Wireless capsule endoscopy detects small bowel ulcers in patients with normal results from state of the art enteroclysis. Am J Gastroenterol. 2003;98:1295-1298. |
| 8. | Riccioni ME, Foschia F, Mutignani M, Perri V, Tringali A, Costamagna G. Small bowel exploration with video capsule endoscopy. Rays. 2002;27:67-72. |
| 9. | Scapa E, Jacob H, Lewkowicz S, Migdal M, Gat D, Gluckhovski A, Gutmann N, Fireman Z. Initial experience of wireless-capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol. 2002;97:2776-2779. |
| 10. | Niv Y, Niv G. Capsule endoscopy: role of bowel preparation in successful visualization. Scand J Gastroenterol. 2004;39:1005-1009. |
| 11. | Niv Y, Niv G, Wiser K, Demarco DC. Capsule endoscopy - comparison of two strategies of bowel preparation. Aliment Pharmacol Ther. 2005;22:957-962. |
| 12. | Albert J, Gobel CM, Lesske J, Lotterer E, Nietsch H, Fleig WE. Simethicone for small bowel preparation for capsule endoscopy: a systematic, single-blinded, controlled study. Gastrointest Endosc. 2004;59:487-491. |
| 13. | Ben-Soussan E, Savoye G, Antonietti M, Ramirez S, Ducrotte P, Lerebours E. Is a 2-liter PEG preparation useful before capsule endoscopy? J Clin Gastroenterol. 2005;39:381-384. |
| 14. | Fireman Z, Paz D, Kopelman Y. Capsule endoscopy: improving transit time and image view. World J Gastroenterol. 2005;11:5863-5866. |
| 15. | Caddy GR, Moran L, Chong AK, Miller AM, Taylor AC, Desmond PV. The effect of erythromycin on video capsule endoscopy intestinal-transit time. Gastrointest Endosc. 2006;63:262-266. |
| 16. | Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005;61:28-31. |
| 17. | Viazis N, Sgouros S, Papaxoinis K, Vlachogiannakos J, Bergele C, Sklavos P, Panani A, Avgerinos A. Bowel preparation increases the diagnostic yield of capsule endoscopy: a prospective, randomized, controlled study. Gastrointest Endosc. 2004;60:534-538. |
| 18. | Sood RR, Joubert I, Franklin H, Doyle T, Lomas DJ. Small bowel MRI: comparison of a polyethylene glycol preparation and water as oral contrast media. J Magn Reson Imaging. 2002;15:401-408. |
| 19. | Basit AW, Newton JM, Short MD, Waddington WA, Ell PJ, Lacey LF. The effect of polyethylene glycol 400 on gastrointestinal transit: implications for the formulation of poorly-water soluble drugs. Pharm Res. 2001;18:1146-1150. |
| 20. | Linden TB, Waye JD. Sodium phosphate preparation for colonoscopy: onset and duration of bowel activity. Gastrointest Endosc. 1999;50:811-813. |
| 21. | Friedman HP, Goldberg JD. Meta-analysis: an introduction and point of view. Hepatology. 1996;23:917-928. |