Published online Feb 14, 2008. doi: 10.3748/wjg.14.969
Revised: December 3, 2007
Published online: February 14, 2008
The incidence of synchronous colorectal and lung cancer is relatively rare. We report three cases of patients with tumors located in the rectum, ascending colon, the lower lobe of the left lung, and the upper lobe of the right lung. Synchronous curative resection of the two lesions was performed in two patients, whereas colectomy was performed in an elderly patient with a poor lung function. Pathological examination showed the colorectal cancer was a moderately differentiated adenocarcinoma and the lung cancer was a squamous cell carcinoma. Surgical treatment and postoperative adjuvant chemotherapy for the lung cancer were different from those for colorectal cancer with pulmonary metastasis. If possible, radical resection should be performed for each cancer when synchronicity is found.
- Citation: Peng YF, Gu J. Synchronous colorectal and lung cancer: Report of three cases. World J Gastroenterol 2008; 14(6): 969-973
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/969.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.969
Curative resection is regarded as a safe and effective treatment for colorectal cancer. The liver is the first and most common site of colorectal cancer metastases. Because of its abundant circulation, the lung is the second most common site of metastases[1]. Coya et al[2] reported that lung metastases develop in 10%-20% of patients with colorectal cancer. Using medical imaging methods (e.g. CT scan), pulmonary metastasis of colorectal cancer almost always shows multiple lesions in both lungs because of the distribution of primary tumor via the circulation. However, about 10% of pulmonary metastases consist of a single solitary pulmonary nodule[2]. When a patient with a current or previous colorectal cancer develops a solitary pulmonary nodule, surgeons always judge it to be a metastasis of colorectal cancer in the first instance. However, in some rare cases, patients can develop synchronous colorectal and lung cancer. In these patients, not only surgical resection but also chemotherapy regimens are quite different from those for colorectal cancer patients with pulmonary metastasis. Therefore, effectively distinguishing synchronous cancers from colorectal cancer with pulmonary metastasis has important implications in therapy for these patients. Although it is reported rarely, three patients with synchronous colorectal and lung cancer were admitted to our department between September 2004 and February 2006.
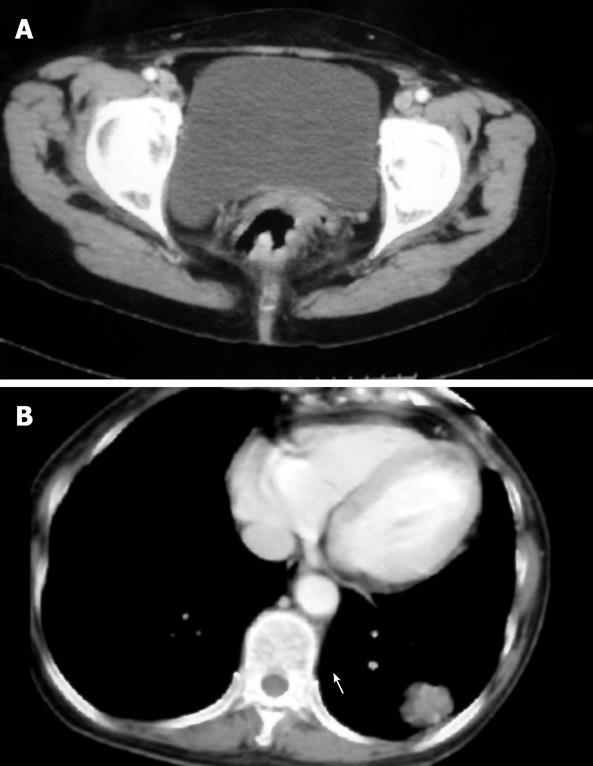
A 63-year-old female patient presented with blood in stools for 4 mo. She had no prior family history of malignancy and no record of carcinogen exposure. A polypoid mass was found 4 cm from the anal verge at colonoscopy. Biopsy of the mass revealed a moderately differentiated adenocarcinoma. CT scan of the pelvic showed the thickening wall of rectum (Figure 1A). Serum carcinoembryonic antigen (CEA) was elevated to 7.9 ng/mL. No metastases were found in the abdomen or chest by CT scan. We performed low anterior resection, and microscopic examination of the surgical resection specimen showed a moderately differentiated adenocarcinoma (2 cm in diameter). The lesion infiltrated the proper muscular layer but not the lymph nodes or vessels. Immunostaining was positive for c-met, p53, p27, cd44, p170, ToPo-II, and MMP-7. According to the National Comprehensive Cancer Network (NCCN) guidelines for rectal cancer, the tumor in this patient was classified as T2N0M0, and not treated with adjuvant chemotherapy after operation. Five months later during routine follow-up, serum CEA was elevated to 28.9 ng/mL. CT scan of the chest showed an irregularly shaped solid mass (3.5 cm in diameter) in the lower lobe of the left lung (Figure 1B), which was an adenosquamous cell carcinoma confirmed by biopsy. The mass in the lung was diagnosed as a metastasis of rectal cancer and a lower lobectomy of the left lung was performed. Microscopic examination of the surgical resection specimen showed an adenocarcinoma (3 cm in diameter) with neuroendocrine differentiation. The lesion invaded the lymph nodes (7/13) and vessels. Immunostaining was positive for CK, EMA, and Syn, but negative for Vim. The final diagnosis was a primary lung cancer. Eighteen months after resection of the lung cancer, the patient died of multiple metastases of the lung cancer.
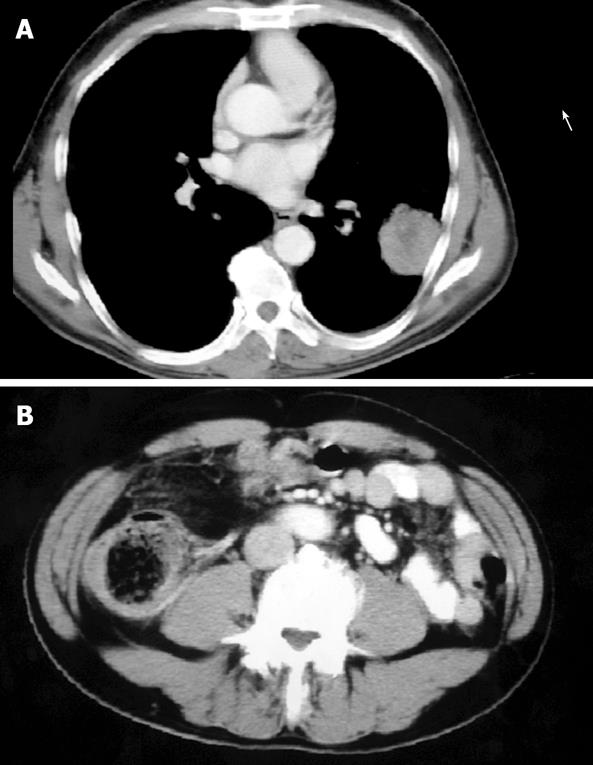
A 60-year-old male patient was admitted for irritant cough in the previous three months. He had no family history of malignancy and alcohol or cigarette abuse, and his dietary habits were normal. CT scan of the chest showed an anomalous round solid mass in the lower lobe of the left lung (Figure 2A). The density of the mass was not uniform, and its margin was blurred. The mass was diagnosed as a primary lung cancer, and we decided to perform a radical lobectomy of the left lung. However, routine B-ultrasonography of the abdomen showed a mass in the right lower abdomen, colonoscopy revealed a polypoid mass in the ascending colon, obstructing the lumen, and a biopsy revealed a moderately differentiated adenocarcinoma. A CT scan of the abdomen showed an intraluminal mass in the ascending colon, the lumen was irregular and stenotic (Figure 2B). Serum CEA was elevated to 7.5 ng/mL. We diagnosed it as colon cancer with metastasis of the lung, and performed a right colectomy and lower lobectomy of the left lung on the same day (Figures 3A and 3B).
Pathological examination of the mass from the colon revealed a moderately differentiated adenocarcinoma, which infiltrated the serosa but not the vessels or lymph nodes (0/12). Immunostaining was positive for CEA, CK20, p53, CDX-2, p27, p170, and Topo-II, while negative for CK7, CD44, and TTF-1.
Pathological examination of the mass (3 cm in diameter) from the lung also revealed a moderately differentiated adenocarcinoma, invading the pleura and lymph nodes (1/11) but not the vessels. Immunostaining was positive for CEA, CK7, p53, CDX-2, and TTF-1, while negative for CK20. The final diagnosis was synchronous colon and lung cancer. After operation, the patient received chemo-therapy (3 wk/cycle) with taxol (75 mg/m2) and oxaliplatin (100 mg/m2) on d 1, with a total of 6 cycles. The patient was still alive 11 mo after operation.
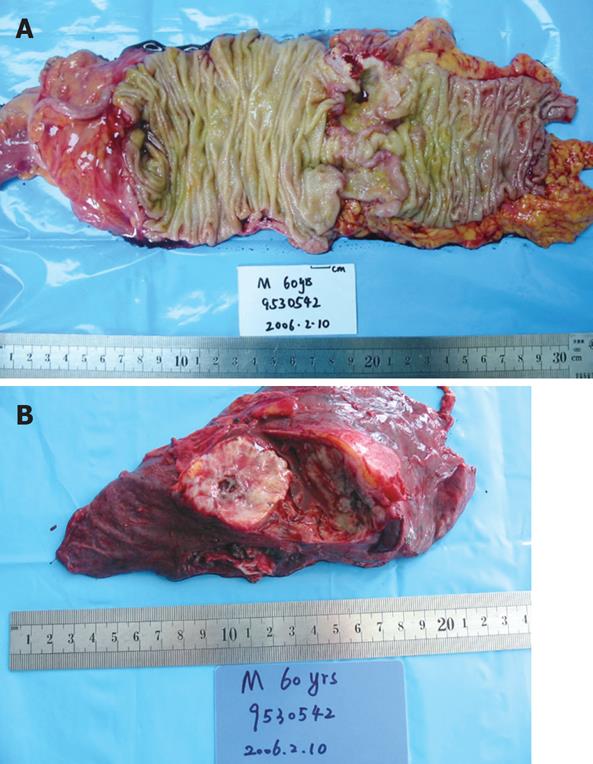
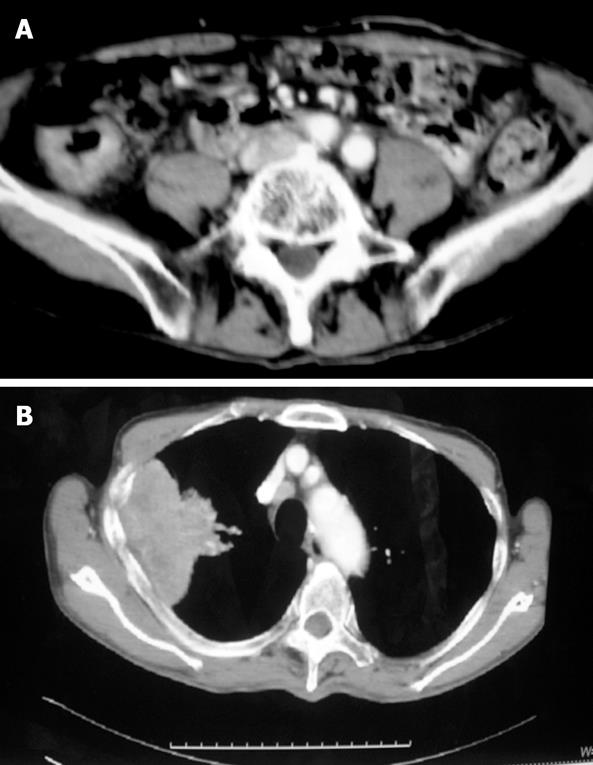
An 81-year-old male patient had heme-positive stools for three months, and was difficult to pass stools for one week. He had no family history of malignancy and alcohol or cigarette abuse. Physical examination was normal, colonoscopy showed an ulcerous mass in the ascending colon, involving the hemi-cycle of the lumen, a biopsy revealed a moderately differentiated adenocarcinoma. B-ultrasonography of the abdomen showed multiple lesions in the liver, and a diagnosis of colon cancer with metastasis of the liver was made. CT scan of the abdomen showed a mass in the ascending colon (Figure 4A). A chest X-ray before operation demonstrated a mass in the upper lobe of the right lung. CT scan of the chest revealed an irregularly shaped mass (5 cm in diameter) in the upper lobe of the right lung, invading the pleura, enlarged mediastinal and hilar lymph nodes (Figure 4B). A CT-guided fine needle biopsy of the mass revealed a poorly differentiated squamous cell carcinoma. Serum CEA was 1.53 ng/mL, CYFRA21-1 and NSE were elevated to 22.90 ng/mL and 14.32 ng/mL. A pulmonary function test showed significant loss of ventilatory function. Since the patient could not tolerate a lobectomy of the lung and had incomplete obstruction of the colon, right hemi-colectomy and biopsy of the lesion on the liver were performed (Figure 5). Pathological examination of the colonic mass (4 cm in diameter) indicated a moderately differentiated adenocarcinoma, invading the serosa but not vessel or lymph nodes (0/5). The liver biopsy revealed a metastatic adenocarcinoma. Immunostaining was positive for c-met, p53, p27, p170, and Topo-II, while negative for CD44. The final diagnosis was colon cancer with multiple metastases in the liver and synchronous primary squamous cell lung cancer. After operation, the patient received chemotherapy (3 wk/cycle) with taxol (175 mg/m2) on d 1, 5-fluorouracil (5-FU) (500 mg/m2) and CDDP (20 mg/m2) on d 1-5, for 3 cycles. The primary lung cancer and liver metastases of the colon cancer were controlled well. The patient was still alive 8 mo after the operation.
Colorectal cancer is the most common cancer, and also most prone to blood-borne metastasis. The liver and lung are the most common sites of metastasis. About 50% of patients develop liver metastases and about 20% develop lung metastases. Therefore, when multiple pulmonary lesions are found in patients with a history of colorectal cancer, most physicians consider them as lung metastases. However, sometimes pulmonary metastases show up as a single solitary lesion. There are a few reports on colorectal cancer with primary lung cancer as a type of multiple primary malignant neoplasm (MPMN) the references is just the Evans et al[3]. Evans et al[3] investigated the incidence of primary lung cancer in 127 281 patients with colorectal cancer and found that the incidence of primary lung cancer is 0.6% (801 cases), while the incidence of synchronous colorectal and lung cancer is much lower. The pathogenesis and biological behavior of synchronous colorectal and lung cancer vs colorectal cancer with pulmonary metastasis are quite different, and so is their treatment. Therefore, when a single solitary pulmonary lesion is found in a colorectal cancer patient, it is important to distinguish between these two possibilities for their appropriate surgical treatment and postoperative adjuvant chemotherapy.
Because of the rare incidence, physicians lack the knowledge of and vigilance towards synchronous colorectal and lung cancer. This situation may lead to misdiagnosis and delay in treatment. When synchronous colorectal and lung cancer are misdiagnosed as colorectal cancer with pulmonary metastasis, adjuvant chemotherapy for colorectal cancer is very unfavorable for the patient.
In our group, two male patients admitted to our department were initially suspected of having a tumor in a single organ. The 60-year-old male patient complained of irritating cough. CT scan revealed a mass in the lower lobe of the lung. Pre-operative abdominal ultrasound examination accidentally found the mass in the right lower abdomen because there were no clear abdominal symptoms, colonoscopy revealed a mass in the ascending colon and adenocarcinoma was confirmed by biopsy. The 81-year-old male patient was admitted to hospital because he felt weak and emaciated with a palpable mass in the right lower abdomen for one week. Colonoscopy found a mass in the ascending colon, which was confirmed as an adenocarcinoma by biopsy. However, conventional preoperative chest radiography showed a suspicious mass in the left upper lobe of the lung, which was confirmed as a squamous cell carcinoma by CT guided fine-needle aspiration. In the female patient with rectal cancer who received the radical LAR procedure, a moderately differentiated adenocarcinoma was confirmed by postoperative pathology. Five months after the operation, conventional chest radiography revealed a nodule in the left lung, which was considered a lung metastasis of rectal cancer and a lower lobectomy of the left lung was performed. The pulmonary nodule was a differentiated adenocarcinoma with neuroendocrine differentiation confirmed by pathology. In the three patients, the symptoms of secondary primary cancer found by conventional preoperative examination or postoperative review were relatively unobvious, suggesting that although the incidence of MPMN is relatively low, when the first primary cancer is found, the surgeon cannot simply be satisfied with a single diagnosis and meticulous systemic examinations of the patient are necessary to avoid misdiagnosis. Further examination of the suspicious lesions should be conducted. If necessary, biopsy can confirm the diagnosis.
When colorectal and pulmonary lesions are found synchronously in a patient, how to distinguish between the pulmonary lesion and primary cancer or the metastasis of colorectal cancer warrants attention.
First, a medical history is helpful for judging the characteristics of pulmonary lesions. Quint et al[4] evaluated 149 patients with extrapulmonary malignant neoplasm and solitary pulmonary nodule and found that a single solitary pulmonary nodule is more likely diagnosed as primary lung cancer than metastasis of other cancers in elderly patients or patients with a history of smoking. They also evaluated the characteristics of solitary pulmonary nodule in patients with a history of colorectal cancer and found the proportion of primary lung cancer and metastases of other cancers is statistically identical. Furthermore, they found that the longer the interval between the detection of primary colorectal cancer and solitary pulmonary nodule is, the more likely the diagnosis of primary lung cancer is. However, in our study, the three patients were non-smoker, and the interval between the detection of colorectal and lung cancer was less than six months.
Second, Quint et al[4] reported that analysis of nodule morphology may provide important information on the lesion etiology and a mass with a smooth margin can be an indication of a benign lesion or a metastasis, whereas a mass with an irregular or blurry margin may be theoretically suggestive of a new lung cancer. Except for patients with melanoma, sarcoma, and testicular carcinoma, the presence of mediastinal lymph node enlargement is strongly suggestive of a primary lung cancer because lymph node enlargement results from metastasis of a primary lung cancer. In our group, the margins of solitary pulmonary nodules in the three patients were all blurry and irregular. On this point, our results are in agreement with the literature reports. However, we found that only one patient had enlarged mediastinal lymph nodes, the other two did not.
Third, sometimes, the characteristics of a pulmonary nodule in a patient with colorectal cancer cannot be determined before operation. In this case, pathological examination of the surgical resection specimen becomes crucial. For example, tumors of different histological findings (e.g. carcinoma and sarcoma) may develop in the same patient. The diagnosis of synchronous MPMN is then without argument. In the 81-year-old male patient, pathology showed the rectal lesion was an adenocarcinoma, whereas the pulmonary lesion was a squamous cell carcinoma. In the female patient, pathology revealed that the rectal lesion was a moderately differentiated adenocarcinoma, whereas the pulmonary lesion was an adenocarcinoma with neuroendocrine differentiation invading the vessels and lymph nodes. Thus, a diagnosis of MPMN was conclusive for these two patients.
However, when similar histological results (e.g. adenocarcinoma) are found in colorectal and pulmonary lesions, the question is whether both lesions are primary cancer or the pulmonary lesion is a metastasis of the colorectal cancer. Microscopy of a conventional pathological section is not enough. Kummer et al[5] used immunohistochemical staining as an effective differential diagnosis method because primary lung cancer shows a CK7(+)/CK20(-) cytokeratin pattern while a metastatic adenocarcinoma shows a CK7(-)/CK20(+) pattern, and found that in their 102 tumor samples, the CK7/CK20 pattern was able to identify lung and colorectal adenocarcinoma in 95% of cases, suggesting that cytokeratin staining is a cost-effective screening tool. Others have reported the use of thyroid transcription factor-1 (TTF-1) for distinguishing between primary and metastatic lung adenocarcinomas[6]. TTF-1 is a common thyroid and peripheral lung antigen, positively stained in primary lung and thyroid adenocarcinoma, whereas metastatic adenocarcinomas from other sites do not express TTF-1. It was reported that over 75% of primary pulmonary adenocarcinomas show strong staining for TTF-1 and a further 11% show weak staining. In their series, apart from a new neuroendocrine tumor, none of the 106 non-pulmonary adenocarcinomas showed staining for TTF-1, indicating that TTF-1 staining is useful in the differential diagnosis of pulmonary adenocarcinoma. The pathology of our 60-year-old male patient showed that colon and pulmonary lesion were a moderately differentiated adenocarcinoma. Therefore, immunohistochemical staining was performed for CK7, CK20 and TTF-1, to distinguish between primary and metastatic lung adenocarcinoma. We found that adenocarcinoma of the colon showed a CK7(-), CK20(+), TTF-1(-) pattern, whereas adenocarcinoma of the lung showed a CK7(+), CK20(-), TTF-1(+) pattern, indicating that it is a primary tumor. Recently, Takashi et al[7] reported that protein TA02 (napsin A), detected by two-dimensional polyacrylamide gel electrophoresis (2-DE), is a valuable marker for discriminating between primary and metastatic lung adenocarcinomas. Using monoclonal antibody to TA02, immunohistochemical staining for TA02 has greater specificity and sensitivity in distinguishing a primary from a metastatic lung adenocarcinoma than TTF-1.
Surgical treatment for patients with synchronous colorectal and lung cancer is quite different from that of colorectal cancer patients with pulmonary metastasis. Pulmonary metastases of colorectal cancer are always multiple lesions in both lungs which are unresectable. Chemotherapy or other palliative therapy is the first choice of treatment for such patients. Curative resection is performed only for patients with single or a few pulmonary metastatic lesions. For patients with synchronous colorectal and lung cancer, if the condition of the patient permits, synchronous curative resection of the two lesions is the first choice. Patient with a poor general state cannot tolerate synchronous curative resection, and the surgeon should first deal with the tumor with a poor prognosis that directly endangers the patient life.
According to the NCCN guidelines, 5-FU and leucovorin combined with oxaliplatin (FOLFOX) or CPT-11 (FOLFIRI) are the first-line chemotherapy regimens for colorectal cancer[8], and cisplatin or carboplatin combined with taxol or docetaxel (TP) or gemcitabine (GP) is the first-line chemotherapy regimen for non-small cell lung cancer[9]. Because few cases of synchronous colon and lung cancer have been reported, a postoperative chemotherapy regimen has not been explicitly specified.
After surgical resection of the synchronous colon and lung cancers in our 60-year-old male patient, we discussed the chemotherapy regimen with the pathologist and oncologist. According to the type of the two primary tumors and stages of pathology, with reference to the NCCN treatment guidelines, a combination of both chemotherapy regimens for primary tumor, a 3-wk/cycle regimen with taxol (175 mg/m2) and oxaliplatin (100 mg/m2) on d 1 was used. After six cycles, no recurrence was found in this patient.
Literature reports indicate that patients with MPMN have a better prognosis than those with a metastasis or recurrent lesion. Tichunsky et al[10] reported that when surgical resection combined with postoperative chemotherapy is performed for colorectal cancer patients with a secondary malignant tumor in another organ, the five-year survival rate is 49%.
Since our 83-year-old male patient had a poor lung function and was unable to tolerate lobectomy of the lung, only colectomy was performed. The other two patients underwent radical resection of both primary tumors. The female patient died of multiple metastases 18 mo after operation, while the 83-year-old male patient with a lung tumor survived for 12 mo and the 60-year-old male patient survived and was disease-free for 13 mo after operation.
Therefore, careful examination, early detection of multiple primary cancers, and active surgical approach can improve the therapeutic effects in MPMN patients.
In conclusion, the incidence of synchronous colorectal and lung cancer is relatively low. When the first primary cancer is found, a meticulous examination of every organ should be conducted until no other malignant neoplasm is found. When suspicious lesions are found, further examination should be performed immediately. If necessary, biopsy can reduce the probability of misdiagnosis and rule out metastatic lesions.
Synchronous radical resection should be performed when the condition of the patient permits. After radical resection, according to the pathological type and stage of the tumors, appropriate adjuvant chemotherapy should be given. The prognosis of synchronous colon and lung cancer is relatively good after careful treatment.
| 1. | Saclarides TJ, Krueger BL, Szeluga DJ, Warren WH, Faber LP, Economou SG. Thoracotomy for colon and rectal cancer metastases. Dis Colon Rectum. 1993;36:425-429. |
| 2. | Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, Kim K, Shim YM. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis. 2007;22:699-704. |
| 3. | Evans HS, Moller H, Robinson D, Lewis CM, Bell CM, Hodgson SV. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut. 2002;50:647-652. |
| 4. | Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology. 2000;217:257-261. |
| 5. | Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86:1884-1887. |
| 6. | Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26:767-773. |
| 7. | Hirano T, Gong Y, Yoshida K, Kato Y, Yashima K, Maeda M, Nakagawa A, Fujioka K, Ohira T, Ikeda N. Usefulness of TA02 (napsin A) to distinguish primary lung adenocarcinoma from metastatic lung adenocarcinoma. Lung Cancer. 2003;41:155-162. |
| 8. | NCCN-National Comprehensive Cancer Network. Practice Guidelines in Oncology: Colon Cancer 2006. Available from: URL: http://www.nccn.org/. |
| 9. | NCCN-National Comprehensive Cancer Network. Practice Guidelines in Oncology: Non-small cell lung Cancer 2006. Available from: URL: http://www.nccn.org/. |
| 10. | Tichansky DS, Cagir B, Borrazzo E, Topham A, Palazzo J, Weaver EJ, Lange A, Fry RD. Risk of second cancers in patients with colorectal carcinoids. Dis Colon Rectum. 2002;45:91-97. |