Published online Feb 14, 2008. doi: 10.3748/wjg.14.918
Revised: December 17, 2007
Published online: February 14, 2008
AIM: To investigate the effects of exogenous melatonin on bacterial translocation and apoptosis in a rat ulcerative colitis model.
METHODS: Rats were randomly assigned to three groups: groupI: control, group II: experimental colitis, group III: colitis plus melatonin treatment. On d 11 after colitis, plasma tumor necrosis factor-α, portal blood endotoxin levels, colon tissue myeloperoxidase and caspase-3 activity were measured. Bacterial translocation was quantified by blood, lymph node, liver and spleen culture.
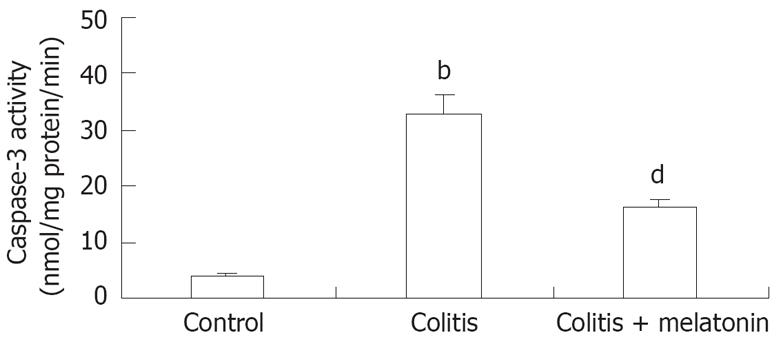
RESULTS: We observed a significantly reduced incidence of bacterial translocation to the liver, spleen, mesenteric lymph nodes, portal and systemic blood in animals treated with melatonin. Treatment with melatonin significantly decreased the caspase-3 activity in colonic tissues compared to that in trinitrobenzene sulphonic acid- treated rats (16.11 ± 2.46 vs 32.97 ± 3.91, P < 0.01).
CONCLUSION: Melatonin has a protective effect on bacterial translocation and apoptosis.
- Citation: Akcan A, Kucuk C, Sozuer E, Esel D, Akyildiz H, Akgun H, Muhtaroglu S, Aritas Y. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J Gastroenterol 2008; 14(6): 918-924
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/918.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.918
Inflammatory bowel diseases (IBDs) are chronic relapsing conditions of unknown etiology with a high morbidity and remain largely incurable. Crohn’s disease (CD) and ulcerative colitis (UC) are collectively termed as IBDs, which are characterized by chronic inflammation in the mucosal membrane at various sites in the gastrointestinal tract with periods of remission and relapse over many years. IBDs are characterized by tissue edema, increased gut epithelial permeability and excessive infiltration of the gut by inflammatory cells from the circulation. This influx results in further tissue-destructive inflammatory processes[1].
The gastrointestinal epithelium forms a barrier that separates the finely regulated homeostasis of the body interstitium from the harsh environment of the intestinal lumen[2]. In IBDs, the intestinal mocosal barrier is disrupted by inflammation and ulceration. In these circumstances, translocation of enteric bacteria and their products through the bowel wall to extra-intestinal sterile sites may occur[3]. Bacterial translocation may cause secondary infection of intra-abdominal inflammatory processes, such as intra-abdominal abscesses, or peritonitis. With regard to endotoxemia and its relationship with severity of disease, increased intestinal epithelial permeability precedes clinical relapse[4], showing that a permeability alteration may be an early event in disease reactivation[2].
The exact pathogenesis of IBD is poorly understood, but there is evidence that IBD involves interactions between immune system, genetic susceptibility and the environment[1]. Recent studies have shown the important role of antiinflammatory and antioxidant agents, including melatonin, in IBDs[56]. Melatonin (5-methoxy-N-acetyltryptamine), a derivative of the essential amino acid tryptophan, is an indolamine hormone with its blood levels varied throughout the day[7]. Melatonin is an agent that promotes sleep and is produced at night by the pineal gland. While produced primarly in the pineal gland, melatonin can also be found in cells of the bone marrow and gastrointestinal tract and plays a fundamental role in the neuroimmuno-endocrine system[8]. In most of the published studies, an antioxidative effect, improved microcirculation and a stimulation of intestinal epithelium may also apply in the beneficial effects of melatonin on the symptoms of colitis induced in rats[59]. The purpose of this study was to determine whether exogenously administered melatonin has any antioxidative and antiinflammatory effects on the impairment of bacterial translocation and apoptosis in an experimental colitis model of rat. To our knowledge, our study is the first one showing the relation between colitis, melatonin, and bacterial translocation.
Male Wistar-albino rats (Erciyes University Hakan Cetinsaya Experimental and Clinical Research Center, Kayseri, Turkey) weighing 225-290 g were fed ad libitum with a nutritionally balanced rodent diet and water. The rats were kept under constant environmental conditions. Only water was provided during the 12 h preceding the experiments. The animals used in this study were handled and treated in accordance with the strict Guiding Principles of the National Institution of Health for Experimental Care and Use of Animals. The Animal Care Committee of the University of Erciyes approved all experimental procedures in this study.
Thirty Wistar-albino rats (28-32 wk old) were used in the study. The rats were divided into 3 groups (10 in each group): groupI(control) = normal animals, group II (colitis) = induction of experimental colitis without further treatment, group III (colitis + melatonin) = induction of experimental colitis plus administration of melatonin.
Colitis was induced as previously described[10]. Briefly, the rats were lightly anesthetized with halothane, and an infant feeding tube (Unoplast A/S DK 330; Hundested, Denmark) fitted onto a blunt 18-gauge needle was inserted rectally. The tip of the tube was placed nearly 8 cm into the colon and 25 mg 2, 4, 6-trinitrobenzene sulphonic acid (Sigma, St. Louis, Missouri) mixed with 0.25 mL 30% ethanol (TNBS-E) was intracolonically instilled. After instillation, the rats were supported at the supine position until recovery from anesthesia to prevent immediate anal leakage of the instillate. The rats in the control group received intracolonic saline. During the 10 subsequent days, the animals in group III were treated with melatonin (10 mg/kg per day) intraperitoneally. Rats in the control and colitis groups received the same volume of steril saline at the same time points. All injections were given after topical application of an antiseptic solution of 10% povidone iodine.
All rats were fed with standard rat chow for 10 d. On the 11th d, all animals underwent operation. Rats were anesthetized by intraperitoneal injection of ketamine-HCl (10 mg/kg; Ketalar®, Eczacibasi, Istanbul, Turkey) and xylazine (3 mg/kg; Rompun® 2%, Bayer, Germany). The abdominal skin was cleansed with povidone-iodine after shaving. All procedures were performed under sterile conditions by a surgeon. The abdomen was opened with a midline incision, 2-mL portal and 2-mL systemic blood samples were collected for biochemical analyses and blood cultures. Samples of liver, spleen, and mesenteric lymph nodes (MLNs) were taken for the determination of bacterial translocation. Colon tissue was sampled for analysis of myeloperoxidase (MPO) and caspase-3 activity as well as for histologic examination. At the end of surgical procedure, all rats were sacrified by overdose ether inhalation.
The levels of serum tumor necrosis factor-α (TNF-α) was measured using a specific enzyme-linked immunosorbent assay kit (The BioSource Rat TNF-α ELISA). The results were expressed as pg/mL.
Plasma endotoxin level in portal venous blood was assayed using quantitative modification of the Limulus-amebocyte-lysate test (Sigma)[11]. The results were expressed as pg/mL.
Portions of the liver, spleen and MLNs were immediately homogenized in saline. Aerobic cultures were performed with blood and eosine-methylene blue agar at 37°C for 24 h. Anaerobic cultures were performed using anaerobic blood agar in Gas-Pak jars at 37°C for 24 h. Colonies were identified with standard microbiologic methods. Blood samples (1 mL) were cultured in tryptic soy medium at 37°C for 7 d. Positive cultures were transferred to blood and EMB agar. Colonies were identified with standard bacteriologic methods.
The distal colon was removed and opened longitudinally. Fecal material was rinsed with a gentle spray of 0.9% saline. The freshly opened colonic segments were pinned out onto a wax block and examined by a pathologist who was blinded to the treatment.
The extent of mucosal damage was assessed using the colon macroscopic scoring system of Wallace et al[12]. Macroscopic inflammation scores were assigned based on clinical features of the colon using an arbitrary scale ranging from 0-10 as follows: 0: normal appearance, 1: hyperemia, 2: hyperemia and thickening without ulceration, 3: ulceration at a single site, 4: two or more sites of ulceration or inflammation, 5: ulceration or inflammation extending > 1 cm along the colon, 6-10: damage covering > 2 cm along the colon, with the score being increased by 1 for each additional centimeter of involvement.
The tissue samples were fixed with 10% formaldehyde solution for 24 h and transferred to 70% ethanol solution. The tissue samples were embedded in paraffin and then cut into 4-&mgr;m thick sections. Histologic examination was performed under light microscope after hematoxylin and eosin staining. A pathologist who was unaware of the animals’groupings evaluated the specimens. Tissue samples were assessed for the presence and activity of colitis as well as the extent of tissue damage using a large number of serial sections. Colonic inflammation was assessed by using a modification of the histopathologic grading system of Macpherson and Pfeifer[13]. The following criteria were used for scoring colonic damage: grade 0: no damage; grade 1: mild mocosal and/or submucosal inflammatory infiltrate and edema: punctuate mucosal erosions often associated with capillary proliferation, muscularis mucosa intact; grade 2: grade 1 changes involving 50% of the specimen; grade 3: prominent inflammatory infiltrate and edema frequently with deeper areas of ulceration extending through the muscularis mucosa into the submucosa, rare inflammatory cells invading the muscularis propria but without muscle necrosis; grade 4: grade 3 changes involving 50% of the specimen; grade 5: extensive ulceration with coagulative necrosis bordered inferiorly by numerous neutrophils and lesser numbers of mononuclear cells, necrosis extending deeply into the muscularis propria; grade 6: grade 5 changes involving 50% of the specimen.
After being scored, a sample of distal colon was frozen for subsequent measurement of MPO activity as an index of granulocyte infiltration[12]. The method described by Bradley et al[14] was used to measure the MPO activity in colon homogenates. The previously frozen colon tissue samples were homogenized for 30 s in 4 mL of 20 mmol/L potassium phosphate buffer (pH 7.4), and centrifuged at 40 000 ×g for 30 min at 4°C. The pellet was resuspended in 4 mL of 50-mmol/L potassium phosphate buffer (pH 6.0), containing 0.5 g/dL hexadecyltrimethyl ammonium bromide. Samples were sonicated for 90 s at full power, incubated in a 60°C water bath for 2 h and centrifuged. The supernatant (0.1 mL) was added to 2.9 mL of 50-mmol/L potassium phosphate buffer (pH 6.0), containing 0.167 mg/mL dianisidine and 0.0005% hydrogen peroxide. Absorbance of 460 nm of visible light (A460) was measured for 3 min. The results were expressed as units of enzyme activity per gram of wet tissue weight (U/g tissue).
The caspase-3 activity ratio was calculated by colorimetric assay (Chemicon International, Temecula, CA). Cells harvested from different treatment groups were lysed and 200 &mgr;g protein was tested for protease activity by adding a caspase-specific substrate peptide, DVED-pnitroaniline. Caspase-3 cleavage of the peptide released the chromophore p-nitroaniline (p-NA), which was quantitated spectro-photometrically at 405 nm. The level of caspase-3 enzymatic activity in the cell lysate was directly proportional to the color reaction. The results are expressed as the fold increase of caspase activity in apoptotic cells relative to their respective controls. In background reactions, no DVED-p-NA substrate was added, and the values obtained were subtracted from experimental results before the fold increase was calculated [15].
The data are presented as mean ± SD. One-way analysis of variance (ANOVA) was used to compare serum TNF-α, endotoxin, and colon MPO and caspase-3 levels among the 3 groups. The Tamhane test was used as the post hoc test for multiple comparisons between groups. Non-parametric Kruskal-Wallis test was used to compare bacterial translocation, and macroscopic and microscopic damage scores. The data were analyzed with the Statistical Package for the Social Sciences (SPSS) for Windows (version 13.0). P < 0.05 was considered statistically significant.
Macroscopic colonic damage: The results of assessment of gross and microscopic colonic injury are listed in Table 1. There was a minimal damage to the surface epithelium, and a mild inflammation of the mucosa but no transmural inflammation in the rats with an evident macroscopic respone to the treatment with melatonin. In the colitis group, the damage comprised broad mucosal ulcers with a surface layer of necrotic slough, accumulation of mesenteric fat, and fibrinous adhesions to the bowel. Macroscopic colonic damage score was the highest in colitis group (mean; 7.1) and was significantly lower in the colitis + melatonin group but higher than in the control group (P < 0.01).
Microscopic colonic damage: Histopathologic evaluation of microscopic colonic damage in the control group was in normal limits. Microscopic examination showed a low level of damage or inflammation in rats treated with melatonin (groups III), whereas there was widespread destruction of the mucosa with transmural infiltration of neutrophils, monocytes, and lymphocytes in rats with colitis (P < 0.05) (Table 1).
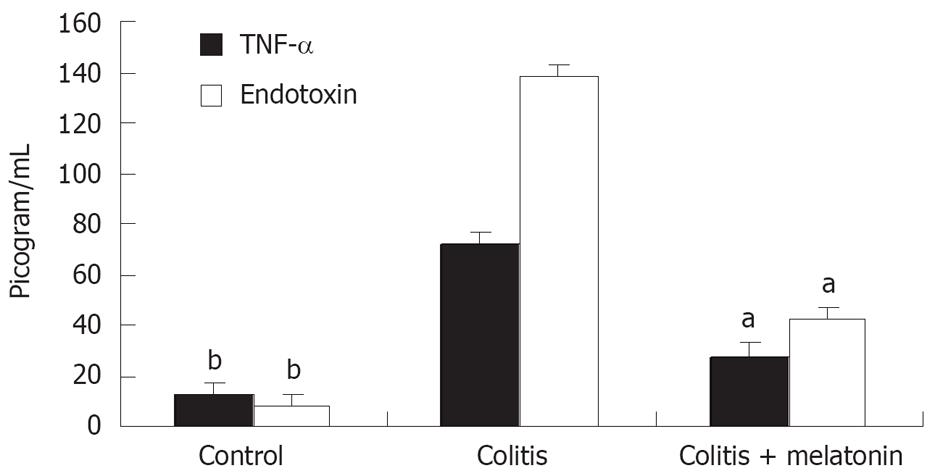
Serum TNF-α and endotoxin levels in the colitis and colitis + melatonin groups were significantly increased on d 11 compared with the control group (P < 0.01, Figure 1). The increase in the colitis + melatonin group was significantly less than that in the colitis group (P < 0.01).
In the control group, no bacterial growth was detected on d 11. TNBS-E colitis caused a significant increase in the frequencies of bacterial translocation in liver, spleen, MLNs, and portal blood. Compared with the colitis group, melatonin decreased the bacterial translocation in liver spleen, MLNs, and portal and systemic blood (P < 0.05 ). The incidence of bacterial translocation in different groups is shown in Table 2. Translocating bacteria were identified as Escherichia coli, Pseudomonas aeruginosa, Enterococus faecalis, and Staphylococcus aureus.
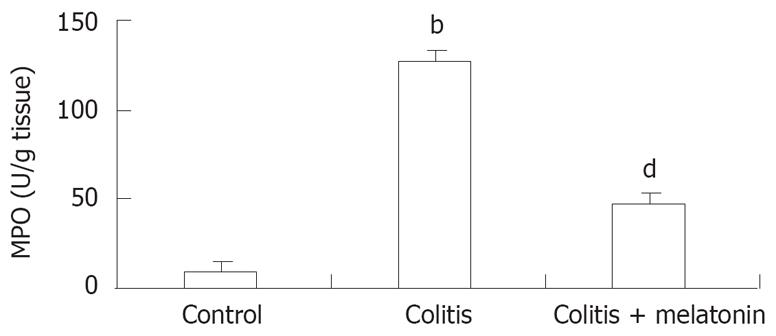
The colon MPO concentrations in rats are shown in Figure 2. The MPO levels in colonic tissue were higher in the colitis group than in the control and melatonin groups (P < 0.001). The colonic MPO activity was significantly lower in the melatonin treatment group than in the TNBS-treatment group (P < 0.001 ).
The caspase-3 level in colonic tissue was significantly higher in the TNBS-E treatment group than in the control group (P < 0.001). Treatment with melatonin significantly decreased the caspase-3 activity compared to the colitis group (P < 0.01, Figure 3).
Ulcerative colitis and CD are the 2 major clinical forms of IBD, clinically characterized by diarrheic attacks, weight loss, and abdominal pain. An increase in incidence has been noticed in the last decade, and the actual prevalence of IBDs is now estimated to be 150 to 200/100.000 people in Europe and North America[16].
Although experimental animal models of colitis are of value in elicing some of the mechanisms of IBDs, the different models differ in their relative use[17]. Most animal models reported lack of certain characteristics of IBDs and model acute events rather than the chronic features typical of IBDs. TNBS colitis has been well characterized and has clinical, biochemical, and pathological similarities to colonic CD. It was reported that animals with TNBS colitis respond to drugs which are useful in treatment of IBDs[12]. This observation, together with the availability of a quantitative scoring system, makes it a useful system for the evaluation of new therapeutic agents. We, therefore, used TNBS-induced colitis, which has the dual advantage of chronicity and acuteness so often in patients with IBDs. Some other advantages of this model of colitis are its reasonable reproducibility and value in assessing the efficacy of therapeutic agents commonly used in the treatment of colitis[1217].
The gastrointestinal tract is continuously exposed to toxins, microbes, microbial products, and other antigens. The intestinal epithelium has several important functions in protecting an organism from the potential deleterious effects of these agents acting as a physical barrier that limits the entry of luminal substances and microorganisms into the lamina propria[18]. Berg et al[19] have described bacterial translocation at the passage of viable bacteria through the epithelial mucosa into the lamina propria, and then to the MLNs and possibly other tissue. It was originally reported that bacterial translocation not only means the migration of intestinal bacteria through the gut (the mechanisms and routes of which have never been identified) but also includes the consequences of this phenomenon, i.e., the infection of extraintestinal tissue with a subsequent systemic inflammatory response, possibly even leading to sepsis with multiple organ dysfunction[20]. The latter indicates that the immune system is no longer capable of eliminating migrating bacteria and/or inflowing toxins and that gut barrier dysfunction may be attributed to both morphological and functional disorders.
There appear to be three main mechanisms that promote bacterial translocation: impaired host defences, physical damage to the intestinal mucosa and disruption of the ecology of the indigenous gut flora resulting in intestinal bacterial overgrowth[19]. At least two of these factors are features of IBD, with an obvious physical disruption of the epithelial barrier by inflammation and ulceration and, as outlined above, disturbed bacterial defences with loss of communal stability and reduced colonization resistance[21]. Several human studies indicate that bacterial translocation also occurs in IBDs. Eade and Brooke[22] demonstrated that portal bacteremia and systemic bacteremia occur in 24% and 1% of patients being submitted to elective colectomy for ulcerative colitis, respectively. They also found that endotoxin plasma levels are positively correlated with clinical disease severity. In addition, bacteria were cultured in liver biopsies from 11% of these patients. Ambrose et al[23] reported that intestinal bacteria are cultured in the ileal serosa from 27% and in the MLNs from 33% of patients undergoing surgery for CD. In our study, both systemic and portal bacteremia were demonstrated, suggestingthat bacteria can be cultured in the MLNs, liver and spleen from TNBS-E colitis group. In this study, all the organ cultures were positive for enteric species, the extent of bacteria translocation from the colonic lumen correlated positively with the colon macroscopic and microscopic score, indicating that disruption of the physical barrier in this model is an important factor for promoting bacterial translocation.
It was previously demonstrated that the antioxidant melatonin exerts beneficial effects on different experimental colitis models of rats[56]. Mazzon et al[24] demonstrated that melatonin treatment significantly reduces the degree of colonic injury, proinflammatory cytokine release and apoptosis. However, the mechanism of the protective effects of melatonin has not been elucidated. The decreased free radical production, immunomodulatory effect and improvement in gut blood flow have to be considered[8]. Melatonin can protect gastrointestinal mucosa gainst damage by stimulating the immune system and fostering microcirculation and epithelial regeneration[25]. The involvement of melatonin in oxygen radical pathophysiology has been confirmed in different colitis models[2627]. As reactive oxygen species play a significant role in the pathogenesis of colitis, melatonin may explain its protective effect on colonic inflammation. The present study showed that melatonin could exert protective effects on colonic inflammation and that reduction of bacterial translocation might be associated with the decreased cell apoptosis.
The extent of immune response can be assassed by measuring the level of MPO as a marker of neutrophil activity in the colon. In the present study, in all TNBS-E treated rats, colonic MPO activity was increased significantly compared to the control group, while melatonin treatment resulted in a decrease in this elevation. This finding is in agreement with earlier investigators who suggested a critical role of neutrophils in colitis[28]. Infiltration and activation of neutrophils lead to excessive generation of proinflammatory cytokines and reactive oxygen species that have been implicated in the pathogenesis of colitis[29]. The decrease in the expression of TNF-α in the melatonin-treated rats supported these findings. Melatonin is a direct antioxidant and also decreases free radical levels by stimulating the activities of enzymes involved in antioxidative defense. Melatonin prevents circulatory failure in rats with endotoxemia by inhibiting the release of TNF-α[30]. TNF-α is a multifunctional cytokine produced primarly by activated monocytes and macrophages and plays a crucial role in the initiation and continuation of mucosal inflammation and immunity[3132]. TNF-α is involved in many cell processes including apoptotic cell death, metabolism, inflammation, thrombosis, and fibrinolysis[33] and induces the production of other cytokines including adhesion molecules, arachidonic acid metabolites, and activation of immune and non-immune cells[34]. It was reported that systemic inhibition of soluble TNF-α with infliximab (chimeric anti-TNF-α mAbs) induces remission in up to 50% of patients with CD and significantly improves clinical symptoms of most patients[35]. Worledge et al[36] reported that administration of TNF-α antibodies can effectively treat experimental rat colitis. In our study, TNF-α levels were parallel to the exacerbated inflammation and increased bacterial translocation in the colitis group. In contrast, TNF-α levels decreased in the melatonin group. At this point, TNF-α may be promising marker for monitoring IBDs activity and response to the treatment. Such findings indicate that blockade of TNF-α signaling has advantages in the treatment of IBDs. The results of our study also show the therapeutic potential of melatonin in human IBDs by affecting the TNF-α levels. In addition to its anti-inflammatory effect, melatonin action in the prevention of colitis may include stimulation of the immun system[37]. Although there is no direct evidence that the effect of melatonin on the immune response is enhanced in the gastrointestinal tract, indirect evidence indicates that melatonin administration to rats significantly increases the number and size of Peyer’s patches[38].
Apoptosis, known as programmed cell death, is an essential physiological process required for the maintenance of tissue homeostasis. Insufficient or excessive cell death can contribute to disease. Although many factors are involved in apoptotic program, caspases have been shown to play a major role in the transduction of apoptotic signals[39]. Recent studies have also shown the induction of apoptosis in different cell lines in response to reactive oxygen species, peroxynitrite and nitric oxide[40]. In the present study, colonic caspase-3 activity was significantly higher in the TNBS-treated rats compared to the control group and treatment with melatonin significantly decreased the caspase-3 activity. Wang et al[41] found that melatonin inhibits hepatic caspase-3 activities and attenuates DNA fragmentation in D-galactosamine-lipopolysaccharid-treated mice. Apoptosis may occur within several hours to several days after injury in some locations, thus, the suppression of cell death is clinically relevant. The role of apoptotic cell death in the inflamed intestine has not been clarified. In UC, the frequency of apoptosis is considerably increased[42] and loss of epithelial cells appears to occur mainly due to apoptosis[43]. Disregulation or inhibition of apoptosis appears to be important in cell proliferation, tissue hyperplasia, and malignant transformation of gastrointestinal epithelia[6]. These findings suggest that epithelial apoptosis may result in an alteration of the epithelial barrier function leading to pathogenic microorganism infiltration. Additonally, apoptosis regulates the lymphocyte population and may inhibit immune response at the inflammatory site[24]. Therefore, it has been proposed that an increased T-cell resistance to apoptosis may contribute to disease progression and mucosal alterations in CD[44]. These results indicate that the protective effects of melatonin against TNBS- induced colitis might be, at least in part, mediated by its anti-apoptotic effects.
In conclusion, melatonin reduces bacterial translocation in TNBS-induced colitis. The anti-inflammatory and anti-apoptotic effects of melatonin can reduce the extent of mucosal damage. Its similar effects in humans remain to be clarified.
Inflammatory bowel disease (IBD), Crohn’s disease and ulcerative colitis have become important health problems in recent years. A growing body of data indicates that oxygen-derived free radicals and nitrogen species have been implicated as mediators of the disruption of the intestinal barrier in IBD, but their molecular targets and pathways have not been defined. It has been shown that the presence of endogenous melatonin in the gastrointestinal tract, and the melatonin’s action as a scavenger of free radicals, have been implicated in the ameliorative effect on experimentaly-induced colitis.
The epithelium of the colon consists of dynamic and rapidly proliferating cells that are profoundly affected by changes in luminal contents and injury. Previous experimental studies have shown that intrarectal instillation of 2, 4, 6-trinitrobenzene sulphonic acid (Sigma, St. Louis, Missouri) mixed with 30% ethanol (TNBS-E) promotes morphologic alterations in the colonic mucosa indicative of atrophy, increases apoptosis, and induces intestinal oxidative stress. Treatment with melatonin can attenuate bacterial translocation and apoptosis as shown in our study by the lower serum levels of tumor necrosis factor-α and endotoxin, the reduced colonic tissue myeloperoxidase and caspas levels.
This study demonstrated that melatonin reduced bacterial translocation in TNBS-induced colitis. The finding supports that exogenous melatonin exerts an anti-inflammatory activity. The mechanism of its anti-inflammatory effect is not entirely clear. In colitis, the frequency of apoptosis is considerably increased and loss of epithelial cells appears to occur mainly due to apoptosis. These results indicate that the protective effects of melatonin on TNBS-induced colitis might be, at least in part, mediated by its anti-apoptotic effects.
The results of the present study further demonstrate the important role of melatonin in regulating epithelial functions. The marked increase of bacterial translocation in postcolitis rats was reversed by melatonin administration due to its anti-inflammatory and anti-apoptotic effects. Further investigations are required to clarify whether melatonin is an effective and safe therapy for IBDs in human beings.
Melatonin, the primary pineal hormone, is also synthesized from tryptophan and secreted in retina, salivary glands, thyroid, liver and intestine, and plays a fundamental role in the neuroimmuno- endocrine system. In the intestine, locally synthesized melatonin is released by enterochromaffin cells. It seems to act as a potent free-radical scavenger rendering it a strong antioxidant.
The experiments are rational and reliable, the statistical methods used are appropriate, results show sufficient experimental evidence, and discussion is well organized and valuable.
| 1. | MacDonald TT, Monteleone G, Pender SL. Recent develo-pments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. |
| 2. | Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282-291. |
| 3. | Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897-901. |
| 4. | Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163-1169. |
| 5. | Nosalova V, Zeman M, Cerna S, Navarova J, Zakalova M. Protective effect of melatonin in acetic acid induced colitis in rats. J Pineal Res. 2007;42:364-370. |
| 6. | Necefli A, Tulumoglu B, Giris M, Barbaros U, Gunduz M, Olgac V, Guloglu R, Toker G. The effect of melatonin on TNBS-induced colitis. Dig Dis Sci. 2006;51:1538-1545. |
| 7. | Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151-180. |
| 8. | Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336-2348. |
| 9. | Marquez E, Sanchez-Fidalgo S, Calvo JR, la de Lastra CA, Motilva V. Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J Pineal Res. 2006;40:48-55. |
| 10. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. |
| 11. | Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Salas A, Forcada P, Laguarda M, Gavalda J, Baena JA, Vilaseca J. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol. 1997;272:G10-G15. |
| 12. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. |
| 13. | MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135-150. |
| 14. | Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206-209. |
| 15. | Avivi-Green C, Madar Z, Schwartz B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int J Mol Med. 2000;6:689-698. |
| 16. | Calkins BM, Mendelhoff AI. The epidemiology of idiopathic inflammatory bowel disease. Inflammatory bowel disease. Baltimore: Williams and Wilkins 1995; 31-68. |
| 17. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. |
| 18. | Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714-1720. |
| 19. | Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403-411. |
| 20. | Stechmiller JK, Treloar D, Allen N. Gut dysfunction in critically ill patients: a review of the literature. Am J Crit Care. 1997;6:204-209. |
| 21. | Gardiner KR, Erwin PJ, Anderson NH, Barr JG, Halliday MI, Rowlands BJ. Colonic bacteria and bacterial translocation in experimental colitis. Br J Surg. 1993;80:512-516. |
| 22. | Eade MN, Brooke BN. Portal bacteremia in cases of ulcerative colitis submitted to colectomy. Lancet. 1969;1:1008-1009. |
| 23. | Ambrose NS, Johnson M, Burdon DW, Keighley MR. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br J Surg. 1984;71:623-625. |
| 24. | Mazzon E, Esposito E, Crisafulli C, Riccardi L, Muia C, Di Bella P, Meli R, Cuzzocrea S. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;41:363-373. |
| 25. | Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189-200. |
| 26. | Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur J Pharmacol. 2001;426:1-10. |
| 27. | Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem. 2002;2:153-165. |
| 28. | Khan I, Hussein SM, Oriowo MA. Studies on the expression and function of beta-3-adrenoceptors in the colon of rats with acetic acid-induced colitis. Pharmacology. 2002;64:98-105. |
| 29. | Blandino II, Otaka M, Jin M, Komatsu K, Odashima M, Konishi N, Sato T, Kato S, Watanabe S. FR167653, a potent suppressant of interleukin-1 and tumor necrosis factor-alpha production, ameliorates colonic lesions in experimentally induced acute colitis. J Gastroenterol Hepatol. 2001;16:1105-1111. |
| 30. | Wu CC, Chiao CW, Hsiao G, Chen A, Yen MH. Melatonin prevents endotoxin-induced circulatory failure in rats. J Pineal Res. 2001;30:147-156. |
| 31. | Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490-495. |
| 32. | Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez-Ramos JC. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol. 1997;62:676-680. |
| 33. | Blam ME, Stein RB, Lichtenstein GR. Integrating anti-tumor necrosis factor therapy in inflammatory bowel disease: current and future perspectives. Am J Gastroenterol. 2001;96:1977-1997. |
| 34. | Kam LY, Targan SR. TNF-alpha antagonists for the treatment of Crohn’s disease. Expert Opin Pharmacother. 2000;1:615-622. |
| 35. | Louis E. The immuno-inflammatory reaction in Crohn’s disease and ulcerative colitis: characterisation, genetics and clinical application. Focus on TNF alpha. Acta Gastroenterol Belg. 2001;64:1-5. |
| 36. | Worledge KL, Godiska R, Barrett TA, Kink JA. Oral administration of avian tumor necrosis factor antibodies effectively treats experimental colitis in rats. Dig Dis Sci. 2000;45:2298-2305. |
| 37. | Pentney PT, Bubenik GA. Melatonin reduces the severity of dextran-induced colitis in mice. J Pineal Res. 1995;19:31-39. |
| 38. | Yanagisawa M, Kachi T. Effects of the pineal hormone on Payers patches in the small intestine. Acta Nat Nippon. 1994;69:522-527. |
| 39. | Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82-93. |
| 40. | Leist M, Fava E, Montecucco C, Nicotera P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur J Neurosci. 1997;9:1488-1498. |
| 41. | Wang H, Xu DX, Lv JW, Ning H, Wei W. Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic liver damage in D-galactosamine-sensitized mice. Toxicology. 2007;237:49-57. |
| 42. | Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Moller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160-167. |