Published online Feb 14, 2008. doi: 10.3748/wjg.14.876
Revised: November 12, 2007
Published online: February 14, 2008
AIM: To investigate the effects and molecular mechanisms of berberine on improving insulin resistance induced by free fatty acids (FFAs) in 3T3-L1 adipocytes.
METHODS: The model of insulin resistance in 3T3-L1 adipocytes was established by adding palmic acid (0.5 mmol/L) to the culture medium. Berberine treatment was performed at the same time. Glucose uptake rate was determined by the 2-deoxy-[3H]-D-glucose method. The levels of IkB kinase beta (IKKβ) Ser181 phosphorylation, insulin receptor substrate-1(IRS-1) Ser307 phosphorylation, expression of IKKβ, IRS-1, nuclear transcription factor kappaB p65 (NF-κB p65), phosphatidylinositol-3-kinase p85 (PI-3K p85) and glucose transporter 4 (GLUT4) proteins were detected by Western blotting. The distribution of NF-κB p65 proteins inside the adipocytes was observed through confocal laser scanning microscopy (CLSM).
RESULTS: After the intervention of palmic acid for 24 h, the insulin-stimulated glucose transport in 3T3-L1 adipocytes was inhibited by 67%. Meanwhile, the expression of IRS-1 and PI-3K p85 protein was reduced, while the levels of IKKβ Ser181 and IRS-1 Ser307 phosphorylation, and nuclear translocation of NF-κB p65 protein were increased. However, the above indexes, which indicated the existence of insulin resistance, were reversed by berberine although the expression of GLUT4, IKKβ and total NF-κB p65 protein were not changed during this study.
CONCLUSION: Insulin resistance induced by FFAs in 3T3-L1 adipocytes can be improved by berberine. Berberine reversed free-fatty-acid-induced insulin resistance in 3T3-L1 adipocytes through targeting IKKβ.
- Citation: Yi P, Lu FE, Xu LJ, Chen G, Dong H, Wang KF. Berberine reverses free-fatty-acid-induced insulin resistance in 3T3-L1 adipocytes through targeting IKKβ. World J Gastroenterol 2008; 14(6): 876-883
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/876.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.876
Berberine is an isoquinoline alkaloid extracted from Rhizoma Coptidis. It was initially used as a heat-clearing and detoxicating agent and an anti-inflammatory drug in clinical practice. Recently, it has been reported that activating the AMP-activated protein kinase (AMPK) pathway is the underlying mechanism for berberine improving insulin resistance, lowering blood sugar, and correcting lipid metabolism disorders[1–4]. A question will be asked if the anti-inflammatory effect of berberine is related to the effect of insulin-resistance improvement. It has been reported that salicylic acid, a traditional anti-inflammatory agent, reverses obesity- and diet-induced insulin resistance by inhibiting IkB kinase β (IKKβ)[56]. This has provided us with a new approach of studying the effect of berberine: is IKKβ a potential target for berberine for improving insulin resistance and relieving inflammation? This study established a model of insulin resistance induced by free fatty acids (FFAs) in 3T3-L1 adipocytes in order to observe the effects of berberine on the expression of inflammation molecules such as IKKβ, nuclear transcription factor kappaB p65 (NF-κB p65) and insulin-signal-transduction related molecules such as insulin receptor substrate-1 (IRS-1), phosphatidylinositol-3-kinase (PI-3K) and glucose transporter 4(GLUT4), with an attempt to confirm that IKKβ is the target for berberine on relieving inflammation and improving insulin resistance.
Berberine (B3251), palmitic acid (PA, P0500), sodium acetylsalicylic acid (As, A5376) and fatty-acid-free bovine serum albumin (FAF BSA, A8806) were all purchased from Sigma (St Louis, MO, USA).
3T3-L1 preadipocytes (obtained from Cell Center of the Institute of Basic Medicines, Chinese Academy of Medical Sciences, China) were grown to confluence in Dulbecco’s minimal essential medium (DMEM; obtained from Gibco Invitrogen, USA) containing 25 mmol/L glucose and 10% fetal bovine serum (FBS; obtained from Gibco Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2. Two days after confluence, cells were placed in DMEM containing 25 mmol/L glucose, 0.5 mmol/L isobutylmethylxanthine (15879), 1 &mgr;mol/L dexamethasone (D4902), 10 &mgr;g/mL insulin (I-6634) (all purchased from Sigma), and 10% FBS for 2 d, and then for 2 d in DMEM that contained 25 mmol/L glucose, 10 &mgr;g/mL insulin, and 10% FBS. Thereafter, cells were maintained in and re-fed every 2 d with DMEM, 25 mmol/L glucose, and 10% FBS until used in experiments 8-12 d after the start , when between 90 and 95% of the cells exhibited an adipocyte phenotype[7].
Fully differentiated 3T3-L1 adipocytes were cultured for 12 h in serum-free DMEM with 0.2% BSA. Then they were cultured for 24 h in DMEM containing 0.5 mmol/L PA and 1% BSA (model group), or for 24 and 48 h in DMEM containing 0.5 mmol/L PA, 10 &mgr;mol/L berberine, 1% BSA (high-dose berberine group, BH24, BH48 group), or in DMEM containing 0.5 mmol/L PA, 1 &mgr;mol/L berberine, 1% BSA for 24 h and 48 h(low-dose berberine group, or BL24, BL48 groups), or cultured in DMEM containing 0.5 mmol/L PA, 5 mmol/L As, 1% BSA for 24 h and 48 h (As group, or As24, As48 group); or in DMEM containing 1% BSA for 24 h (normal group).
3T3-L1 preadipocytes (5 × 105/well) were differentiated to adipocytes in a 24-well plate. After serum-starvation in 0.2% BSA DMEM overnight, the cells were incubated in 0.2% BSA DMEM containing 0.5 mmol/L PA or (and) 1 &mgr;mol/L, 10 &mgr;mol/ L Ber or 5 mmol/L aspirin for 24 or 48 h. Then, the cells were incubated in 1 mL Krebs/Ringer phosphate (KRP)/HEPES (131.2 mmol/L NaCl, 4.71 mmol/L KCl, 2.47 mmol/L CaCl2, 1.24 mmol/L MgSO4, 2.48 mmol/L Na3PO4, 10 mmol/L HEPES, pH 7.4) with or without 100 nmol/L insulin for 30 min at 37°C, after being washed three times in KRP/HEPES buffer. Next, the cells were incubated in 1 mL KRP/HEPES containing 0.5 &mgr;Ci/mL 2-deoxy-D-[3H] glucose (purchased from Beijing Yuanzi Hi-Tech, China) for 10 min at 37°C. Finally, the cells were washed three times in ice-cold PBS and solubilized in 1 mL 0.1 mol/L NaOH for 2 h. Radioactivity was determined by liquid scintillation spectrometry. Non-specific deoxyglucose uptake was measured in the presence of 20 &mgr;mol/L cytochalasin B (Sigma), and specific glucose uptake was detected from the subtracted total uptake. Three replicate wells were set up and each experiment was repeated three times[8]. Additionally, a Cell Counting Kit-8 (CCK-8) was employed for the monitoring of the number and viability of the cells[9].
3T3-L1 preadipocytes were grown and differentiated into adipocytes in 35-mm culture dishes. After serum-starvation in 0.2% BSA DMEM overnight, the cells were incubated in 0.2% BSA DMEM containing 0.5 mmol/L PA or (and) 1 &mgr;mol/L, 10 &mgr;mol/L Ber or mmol/L Aspirin for 24 or 48 h. Then, whole-cell lysate was made in lysis buffer (1% Triton X-100, 50 mmol/L KCl, 25 mmol/L HEPES, pH 7.8, 10 &mgr;g/mL leupeptin, 20 &mgr;g/mL aprotinin, 125 &mgr;mol/L dithiothreitol (DTT), 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L sodium orthovanadate) by sonication. For NF-κB p65 detection, cytosolic and nuclear fractions were separated (first buffer: 10 mmol/L HEPES pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 1.5 mmol/L MgCl2, 0.1% NP4O, 1 mmol/L DTT; second buffer: 20 mmol/L HEPES pH 7.9, 420 mmol/L NaCl, 0.1 mmol/L EDTA, 1.5 mmol/L MgCl2, 25% glycerol, 1 mmol/L DTT, 0.5 mmol/L PMSF. Protein concentrations were measured by BCA Protein Assay kit (Pierce, USA).The protein (50 &mgr;g) in 50 &mgr;L reducing sample buffer was boiled for 5 min, and resolved by SDS-PAGE for 2 h at 110 V. Then, the protein was transferred onto a polyvinylidene difluoride (PVDF) membrane. After transfer, the PVDF membrane was washed with 25 mL Tris-buffered saline (TBS) for 5 min at room temperature. The membrane was incubated in 25 mL blocking buffer (1 × TBS, 0.1% Tween-20 with 5% non-fat dry milk) for 2 h at room temperature, and then washed three times for 5 min each with 15 mL TBS/0.1% Tween-20 (TBS/T). The membrane was incubated with primary antibody including IKKβ antibodies, phosphorylated IKKβ Ser181 antibodies, phosphorylated IRS-1 Ser307 antibodies (Cell Signaling, USA), IRS-1 antibodies, PI-3K p85 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), GLUT4 antibodies (R&D Systems, USA) NF-κB p65 antibodies (Neomarkers, USA), β-actin antibodies (Lab Vision, USA) (at the appropriate dilution) in 10 mL primary antibody dilution buffer with gentle agitation overnight at 4°C, and then washed three times for 5 min each with 15 mL 10 ×TBS/0.1% TBS/T. The membrane was incubated with HRP-conjugated secondary antibody (1:3000; Pierce) in 10 mL blocking buffer with gentle agitation for 2 h at room temperature, and washed three times for 5 min each with 15 mL TBS/T. Immunoreactive bands were detected with the ECL kit (Pierce) and immunoreactive bands on autoradiography films were scanned (Bio-Rad). Intensity of the immunoblot signal was analyzed quantitatively using Quantity One software (Bio-Rad).
3T3-L1 preadipocytes were differentiated into adipocytes in a six-well plate. After serum-starvation in 0.2% BSA DMEM overnight, the cells were incubated in 0.2% BSA DMEM containing 0.5 mmol/L PA or (and) 10 &mgr;mol/L Ber or 5 mmol/L aspirin for 24 or 48 h. Cells were harvested and coverslips were fixed with fixed liquid (dehydrated alcohol: acetone; 1:1 v/v) for 10 min and 1% Triton X-100 for 5 min. After completely rinsing with water and washing with PBS, rabbit-anti-human NF-κB p65 antibody (1:100, 50 &mgr;L) was added drop wise and stored at 4°C overnight. The following day, after washing with PBS, Cy-3-labeled sheep-anti-rabbit IgG was added drop wise, and the samples were incubated in a wet box in the dark at 37°C for 30 min. After washing with PBS in the dark, diamidino phenylindole (DAPI) solution was added drop wise to counterstain the nucleus for 30 s (in the dark at room temperature, about 37°C). After washing with PBS and distilled water, 50% buffered glycerol was used for coverslip sealing. Image acquisition was achieved using an Olympus FV500 fluorescent microscope. An immersion objective lens was used to observe the samples. The excitation wavelengths of CY-3 and DAPI were 552 and 358 nm, respectively. The emission wavelengths were 565 and 460 nm, respectively. Ten randomly selected areas were taken from each coverslip to account for variations in the expression levels of NF-κB p65. Images were analyzed quantitatively by the HMIAS-2000 Imaging System, China.
Data are expressed as means ± SD. Statistical and graphical analysis was performed with SPSS version 10.0 software. Analyses were performed using an independent-samples t test where appropriate.
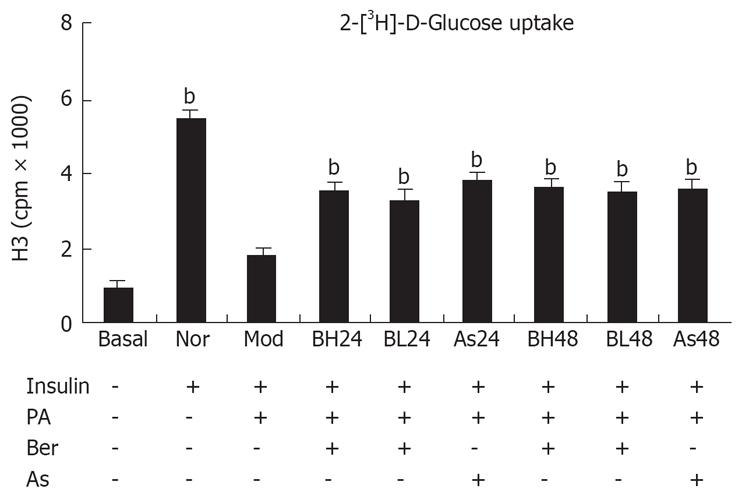
We used 3T3-L1 adipocytes as a cellular model to analyze the insulin signaling pathway. 3T3-L1 adipocytes were treated with PA (0.5 mmol/L, 24 h) to induce insulin resistance. Insulin-induced glucose uptake was measured to determine insulin sensitivity. The results showed insulin-induced glucose uptake was 5.6 times higher than that in the normal group, but was inhibited by as much as 67% after incubation with PA for 24 h. This is consistent with reports FFA induces insulin resistance in cell culture[1011]. However, intervention with berberine or aspirin reversed the condition completely. Insulin-induced glucose uptake was increased by 82% and 93% after intervention with 1 and 10 &mgr;mol/L berberine for 24 h, and increased by 93% and 101% after intervention with berberine for 48 h, respectively. The data was performed in a dose- and time-dependent manner. In addition, the insulin-induced glucose uptake was increased by 109% and 97%, respectively, in the aspirin groups (Figure 1). The viability and number of cells might have decreased because of the toxic effects of 48-h aspirin treatment (Table 1).
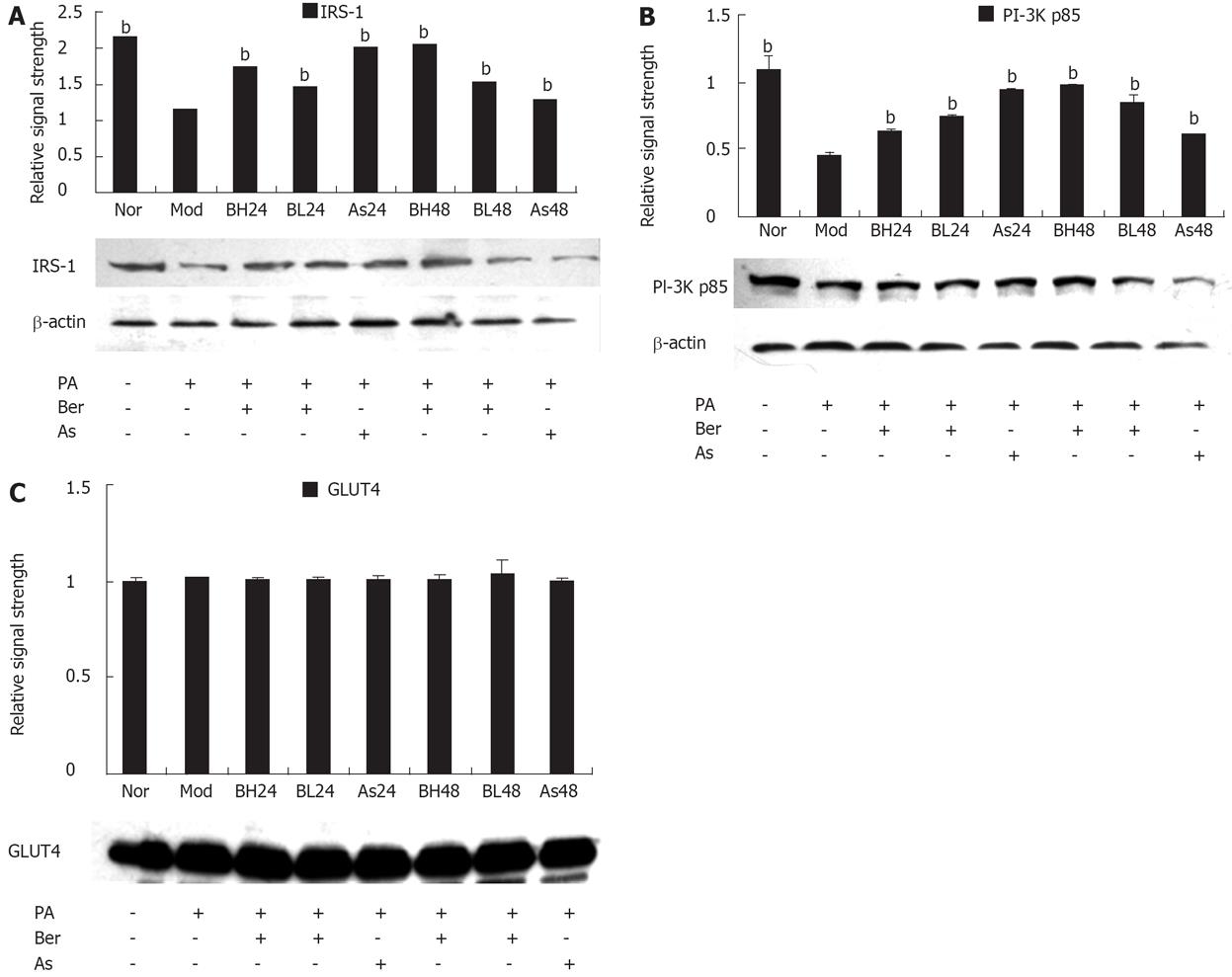
To understand the role of insulin signal transduction proteins in the mechanism by which berberine acts on FFA-induced insulin resistance, IRS-1, PI-3K p85 and GLUT4 protein abundance were detected with IRS-1, PI-3K p85 and GLUT4 antibodies by immunoblotting in 3T3-L1 adipocytes. The results showed that expression of IRS-1 and PI-3K p85 protein was reduced after incubation with FFA (0.5 mmol/L PA, 24 h) (P < 0.01) and this reduction was inhibited by berberine (1 or 10 &mgr;mol/L) or aspirin (5 mmol/L). The data was still performed in a dose- and time-dependent manner (P < 0.01) (Figure 2A and B). However, there was no change in the expression of GLUT4 protein after the intervention of FFA, berberine and aspirin (Figure 2C).
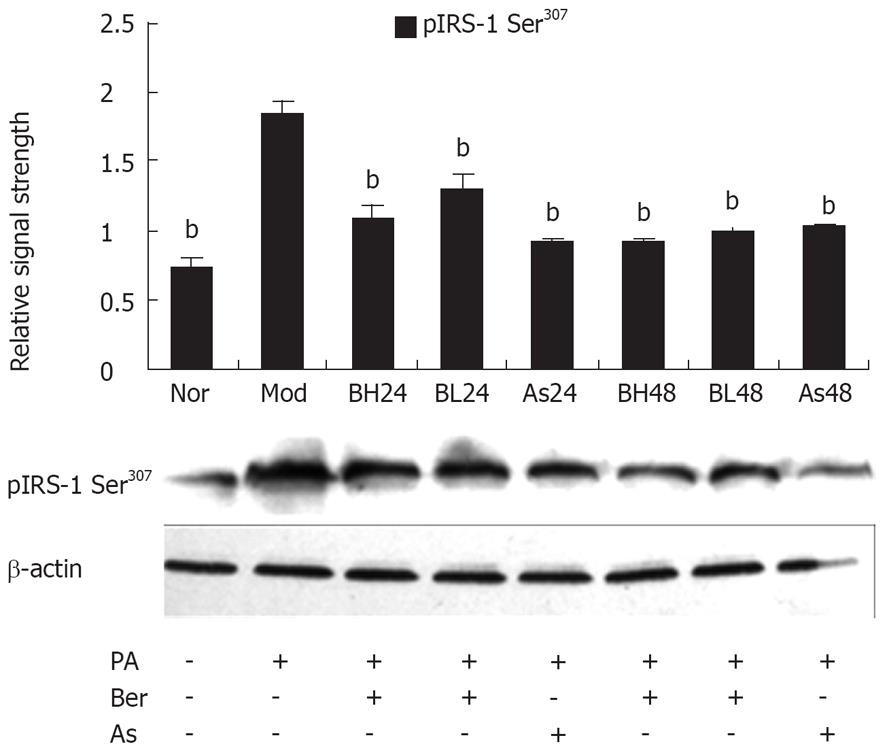
Serine phosphorylation precedes IRS-1 degradation[12–14] and Ser307 phosphorylation of IRS-1 has become a molecular indicator of insulin resistance[15–18], therefore, we investigated the effect of berberine on IRS-1 Ser307 phosphorylation with phosphorylation-specific IRS-1 Ser307 antibody by immunoblotting. In 3T3-L1 adipocytes, PA (0.5 mmol/L, 24 h) may have induced IRS-1 Ser307 phosphorylation and berberine (1 or 10 &mgr;mol/L) or aspirin (5 mmol/L) were found to reduce IRS-1 Ser307 phosphorylation, and this reduction by berberine was dose- and time-dependent. These results suggest that Ser307 phosphorylation contributes to IRS-1 degradation and berberine inhibits FFA-induced IRS-1 Ser307 phosphorylation in 3T3-L1 adipocytes (Figure 3).
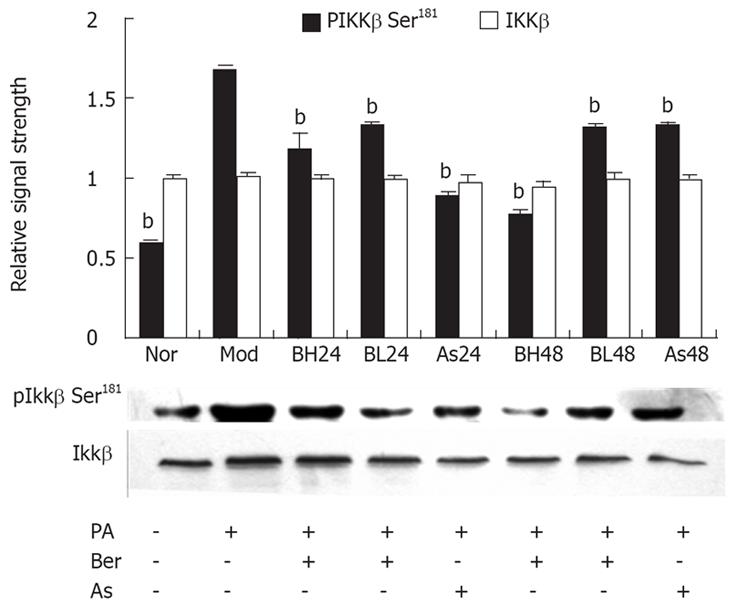
It was reported that Ser307 of IRS-1 can be phosphorylated by either IKK or JNK[1519], and IKK is more sensitive to FFAs. In mammalian cells, phosphorylation of Ser181 at the activation loop is essential for activation of the catalytic activity of IKK[2021]. In this study, we investigated an effect of berberine on IKKβ serine181 phosphorylation and protein abundance of IKKβ with the phosphorylation-specific IKKβ Ser181 antibody and IKKβ antibody by immunoblotting. In 3T3-L1 adipocytes, PA (0.5 mmol/L, 24 h) may induce IKKβ Ser181 phosphorylation; berberine (1 &mgr;mol/L, 10 &mgr;mol/L) or aspirin (5 mmol/L) were found to reduce IKKβ Ser181 phosphorylation, with the the reduction by berberine in a dose- and time-dependent manner (P < 0.01). However, IKKβ protein abundance was unchanged during this study. These results suggest Ser307 phosphorylation of IRS-1 is associated with activation of IKKβ, and that berberine inhibits FFA-induced IKKβ Ser181 phosphorylation in 3T3-L1 adipocytes (Figure 4).
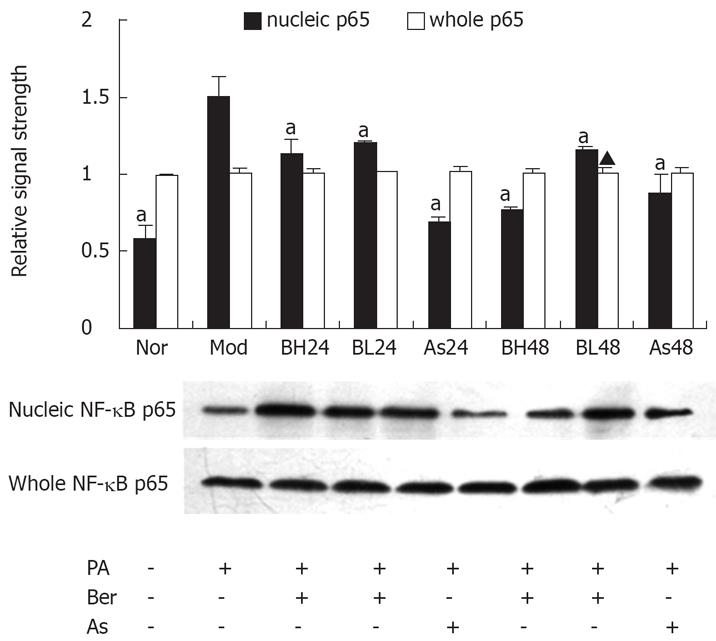
The NF-κB/Rel transcription factors are present in the cytosol in an inactive state, combined with the inhibitory IkB proteins. The key step in NF-κB nuclear translocation is mediated by IKKβ. In this study, we examined the effect of berberine on NF-κB p65 protein abundance obtained from whole-cell extracts and nuclear extracts with NF-κB p65 antibody, by immunoblotting. The results showed that, in 3T3-L1 adipocytes, PA (0.5 mmol/L, 24 h) may have induced nuclear NF-κB p65 protein, and berberine (1 or 10 &mgr;mol/L) or aspirin (5 mmol/L) were found to reduce expression of NF-κB p65 protein, and this reduction by berberine was dose- and time-dependent (P < 0.05). However, whole NF-κB p65 protein abundance was unchanged during this study (Figure 5).
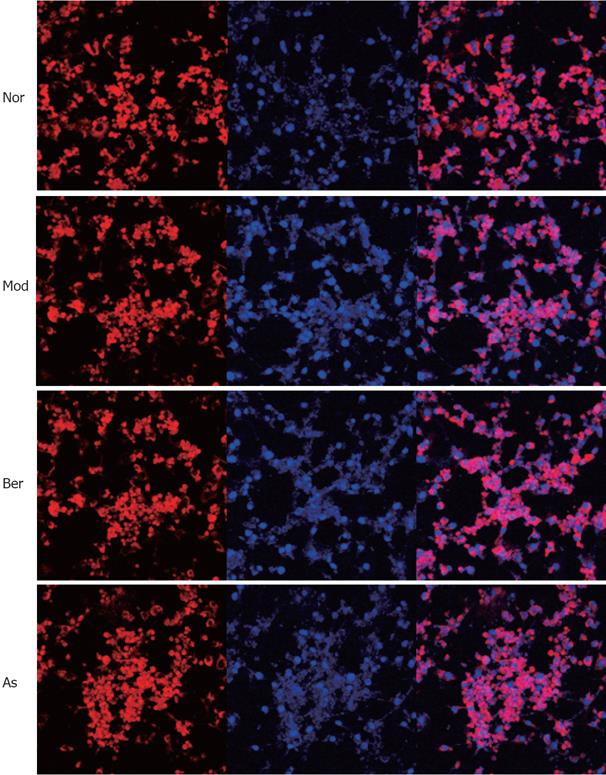
To test the hypothesis that berberine inhibits expression of FFA-induced NF-κB p65 protein in 3T3-L1 adipocytes, immunofluorescence by LSCM was used to investigate the distribution of NF-κB p65. Nuclei were stained with DAPI (blue) and NF-κB p65 was stained with CY-3 (red). The confocal superimposition of the two fluorescence stains yielded a pink color, which indicated that NF-κB p65 was translocated into the nuclei. The results showed that, without FFA stimulation, NF-κB p65 was predominately found in the cytoplasm, and imparted a red color (normal group), while with FFA stimulation, NF-κB p65 was translocated to the nucleus, and showed a pink color (model group). The addition of 10 &mgr;mol/L berberine or 5 mmol/L aspirin clearly inhibited the FFA-induced NF-κB p65 translocation; there was hardly any nuclear p65 staining found, and in the figure, both red and pink colors can be observed (indicating berberine and aspirin), which is consistent with the Western blot analysis results (Figure 6).
Berberine is an isoquinoline alkaloid extracted from Rhizoma Coptidis, and it was initially used as a heat-clearing and detoxicating agent and as an anti-inflammatory drug in clinical practice. Further pharmacological studies have found that the effects of berberine include improving insulin resistance, lowering blood sugar, and correcting disorders of lipid metabolism[1–4]. Based on the effects of berberine on diabetes and the new notion that type 2 diabetes is essentially a disease of chronic inflammation, we make hypothesis if the anti-inflammation effect of Ber is related to the effect of insulin-resistance improvement.
Recently, it has been reported that salicylic acid, a traditional anti-inflammatory agent, reverses obesity- and diet-induced insulin resistance by inhibiting IKKβ[56]. Many studies have shown insulin signal transduction proteins are regulated, not only by tyrosine phosphorylation, but also by Ser/Thr phosphorylation. IKKβ, a Ser/Thr protein kinase, is closely related to the development of insulin resistance and is known to be a new target for the improvement of insulin resistance[5]. Is IKKβ a potential molecular target for berberine for improving insulin resistance and relieving inflammation?
In our previous studies, we have established a murine model of insulin resistance by injection of small doses of streptozotocin via the tail vein plus oral administration of high-fat and high-caloric diet. After treatment with berberine for 10 wk, the levels of fasting blood glucose and insulin were substantially lowered; the oral glucose tolerance test was conspicuously improved; serum inflammatory factors IL-1β, IL-6, TNF-α, acute-phase reactive protein (CRP), and adhesion molecules (ICAM-1, VCAM-1) were obviously lowered; and at the same time, tyrosine phosphorylation of insulin receptor β subunit, tyrosine phosphorylation of IRS-1, expression of IRS-1, GLUT4, PI-3K protein, and translocation of GLUT4 protein were significantly elevated in adipose and muscular tissues, compared with those in the model group. This suggests berberine reduces inflammation, lowers blood glucose and improves insulin resistance[22–24].
In the present study, we investigated the underlying mechanisms of the effects of berberine on insulin resistance and inflammation in vitro. Insulin resistance in 3T3-L1 adipocytes was induced by incubation with PA (0.5 mmol/L, 24 h), as judged by a 67% decrease in insulin-stimulated glucose uptake. This was reversed by prior incubation of cells with berberine or aspirin. The expression of IRS-1 and PI-3K p85 proteins was reduced after PA incubation and this reduction was inhibited by intervention with berberine (1 or 10 &mgr;mol/L) or aspirin (5 mmol/L). However, there was no change in GLUT4 abundance after incubation with FFAs, berberine and aspirin. The results suggest inhibition of insulin-induced glucose uptake is caused by reduction of IRS-1 and PI-3K, not GLUT4. Thus, IRS-1 and PI-3K p85 protein, but not GLUT4, might be involved in the effects of berberine on insulin resistance in 3T3-L1 adipocytes.
It has been shown Ser307 phosphorylation contributes to IRS-1 degradation in hepatocytes in response to insulin[25]. In our study, PA may have increased IRS-1 Ser307 phosphorylation, while berberine or aspirin were found to reduce IRS-1 Ser307 phosphorylation. It can be inferred that the same mechanism contributes to FFA-induced degradation of IRS-1 in adipocytes, and Ser307 phosphorylation of IRS-1 was inhibited by berberine. Since Ser307 of IRS-1 can be phosphorylated by IKK[1519] and phosphorylation of Ser181 at the activation loop is essential for activation of the catalytic activity of IKK[2021], we observed the expression of IKKβ Ser181 phosphorylation in 3T3-L1 adipocytes. PA may have increased IKKβ Ser181 phosphorylation, and this was reversed by prior incubation of cells with berberine or aspirin. It was demonstrated that IKKβ Ser181 phosphorylation may contribute to IRS-1 Ser307 phosphorylation in response to FFAs, and berberine inhibited FFA-induced IKKβ Ser181 phosphorylation in 3T3-L1 adipocytes.
It is known the NF-κB/Rel transcription factors are present in the cytosol in an inactive state, complexed with the inhibitory IkB proteins, and that NF-κB nuclear translocation is mediated by IKKβ[2627]. In our study, we detected NF-κB p65 protein in whole-cell and nuclear extracts, and investigated the distribution of NF-κB p65. It has been shown that in 3T3-L1 adipocytes, PA may increase the expression of NF-κB p65 protein, while berberine or aspirin reduce the expression of NF-κB p65 protein. However, NF-κB p65 protein abundance was not changed during this study. LSCM demonstrated significant translocation of NF-κB p65 into the nucleus after stimulation with PA. The translocation was inhibited by berberine or aspirin, which is consistent with the results of Western blot analysis. Taken together, the data suggest that berberine inhibits translocation of NF-κB p65 into the nucleus in 3T3-L1 adipocytes.
In summary, our study demonstrated potential links between chronic inflammation and insulin resistance. IKKβ may be an important target for this link. Increased IKKβ activity promotes insulin resistance, while reduction in IKKβ activity significantly improves insulin sensitivity. In the signaling pathway, FFAs activate IKKβ, which leads to Ser307 phosphorylation in IRS-1 and translocation of NF-κB p65 into the nucleus[2829]. On the one hand, serine phosphorylation is responsible for a reduction in tyrosine phosphorylation of IRS-1, which leads to a reduction in IRS-1 protein and insulin resistance. On the other hand, translocation of NF-κB p65 into the nucleus may enhance the production of inflammatory molecules such as TNFα. The positive feedback loop may perpetuate a cycle of low-level inflammatory signaling that leads to insulin resistance. Our findings were consistent with those reported by Gao et al[30]. Thus, inhibitors of IKKβ, such as aspirin and berberine, through the same signaling pathway, have significant effects on insulin resistance. Therefore, we conclude berberine might achieve its anti-inflammatory and insulin-resistance-improving effects by inhibiting phosphorylation of IKKβ Ser181.
Berberine was initially used as a heat-clearing and detoxifying agent and an anti-inflammatory drug in clinical practice. Further pharmacological studies have found the effects of berberine include improving insulin resistance, lowering blood sugar and correcting disorders of lipid metabolism in vitro and in vivo. It has been shown that type 2 diabetes is essentially a disease of chronic inflammation. The question remains if the anti-inflammatory effect of berberin is related to the effect of insulin resistance improvement.
Recently, it has been reported that salicylic acid, a traditional anti-inflammatory agent, may reverse obesity- and diet-induced insulin resistance by inhibiting IKKβ. Many studies have shown insulin signal transduction proteins are regulated, not only by tyrosine phosphorylation, but also by Ser/Thr phosphorylation. IKKβ, a Ser/Thr protein kinase, is closely related to the development of insulin resistance and is known as a new target for the improvement of insulin resistance. Is IKKβ a potential molecular target for berberine for improving insulin resistance and relieving inflammation?
Our research has established a model of insulin resistance induced by FFA in 3T3-L1 adipocytes, and we have observed the effects of berberine on the expression of inflammatory molecules such as IKKβ, NF-κB and insulin-signal-transduction-related molecules such as IRS-1, PI-3K and GLUT4. These results showed IKKβ may be an important target for potential links between chronic inflammation and insulin resistance. Berberine might achieve its anti-inflammatory and insulin-resistance-improving effects by inhibiting phosphorylation of IKKβ Ser181.
Our study showed IKKβ may be a potential molecular target for berberine when improving insulin resistance and relieving inflammation. The use of berberine as an inhibitor of IKKβ provides us with a new approach for the treatment of non-infectious chronic diseases, such as type 2 diabetes, obesity, hypertension, atherosclerosis and ischemic cerebrovascular disease.
IKKβ: IKK is a multi-subunit protein kinase. There is strong biochemical and genetic evidence that the IKK complex consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ. However, of the two catalytic subunits, only IKKβ is essential for NF-κB activation in response to pro-inflammatory stimuli. Many studies have shown that IKKβ, a Ser/Thr protein kinase, is closely related to the development of insulin resistance, and is known as a new target for the improvement of insulin resistance.
This study investigated the influence of berberine on FFA-treated 3T3-L1 cells. Similar data have not been published so far and the figures are clearly presented.
| 1. | Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256-2264. |
| 2. | Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439-1443. |
| 3. | Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25:496-502. |
| 4. | Ko BS, Choi SB, Park SK, et al. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–1437. |
| 5. | Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673-1677. |
| 6. | Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437-446. |
| 7. | Nelson BA, Robinson KA, Buse MG. High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes. 2000;49:981-991. |
| 8. | Romero R, Casanova B, Pulido N, Suarez AI, Rodriguez E, Rovira A. Stimulation of glucose transport by thyroid hormone in 3T3-L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J Endocrinol. 2000;164:187-195. |
| 9. | Takeuchi A, Mishina Y, Miyaishi O, Kojima E, Hasegawa T, Isobe K. Heterozygosity with respect to Zfp148 causes complete loss of fetal germ cells during mouse embryogenesis. Nat Genet. 2003;33:172-176. |
| 10. | Storz P, Doppler H, Wernig A, Pfizenmaier K, Muller G. Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem. 1999;266:17-25. |
| 11. | Van Epps-Fung M, Williford J, Wells A, Hardy RW. Fatty acid-induced insulin resistance in adipocytes. Endocrinology. 1997;138:4338-4345. |
| 12. | Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359-1364. |
| 13. | Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001;276:40362-40367. |
| 14. | Zhande R, Mitchell JJ, Wu J, Sun XJ. Molecular mechanism of insulin-induced degradation of insulin receptor substrate 1. Mol Cell Biol. 2002;22:1016-1026. |
| 15. | Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531-1537. |
| 16. | Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115-48121. |
| 17. | Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896-2902. |
| 18. | Hirosumi J, Tuncman G, Chang L, Gorgun C, Uysal K, Maeda K, Karin , M , and Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. |
| 19. | Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278:24944-24950. |
| 20. | Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860-866. |
| 21. | Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309-313. |
| 22. | Xiao YL, Lu FE, Xu LJ, Leng SH, Wang KF. Protective effects of Huanglian Jiedu decoction on vascular endothelial function in type 2 diabetic rats. Zhongguo Zhongyao Zazhi. 2005;30:1767-1770. |
| 23. | Ye AL, Lu FE, Xu LJ. Effects of huanglian jiedu decoction on signal transduction of insulin receptor and insulin receptor substrate in adipose tissue of insulin resistant rats. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:909-912. |
| 24. | Tan Y, Lu FE, Xu LJ, Leng SH, Wang KF. Effect of Huanglian Jiedu decoction on serum inflammatory mediators and marks of type 2 diabetic rats. Zhong Guo Yi Yuan Yao Xue Za Zhi. 2005;25:1113-1115. |
| 25. | Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003;278:8199-8211. |
| 26. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. |
| 27. | Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839-1845. |
| 28. | Park E, Wong V, Guan X, Oprescu AI, Giacca A. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol. 2007;195:323-331. |
| 29. | Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934-945. |
| 30. | Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024-2034. |