Published online Feb 7, 2008. doi: 10.3748/wjg.14.747
Revised: November 30, 2007
Published online: February 7, 2008
AIM: To evaluate the role and outcome of conventional surgery in the treatment of pyogenic liver abscess in the modern era of minimally invasive therapy.
METHODS: The medical records of thirteen patients with pyogenic liver abscess who underwent surgical treatment between January 1995 and December 2002 were retrospectively reviewed to determine the clinical presentation, indication and nature of surgery, and outcome of surgery.
RESULTS: The patients were predominantly women (10/13) with a mean age of 65 ± 17 years. Their main presenting symptoms were abdominal pain (100%) and fever (77%). The aetiologies included biliary (n = 6), cryptogenic (n = 3), portal (n = 2), and trauma (n = 2). Seven patients underwent percutaneous drainage as the initial treatment. Of these, three patients developed peritonitis secondary to peritoneal spillage. Another four patients failed to respond because of multiloculation. Salvage surgery was required in these patients. Six patients proceeded to straight laparotomy: two had marked sepsis and multiloculated abscess that precluded percutaneous drainage, and four presented with peritonitis of uncertain pathology. Surgical procedures included deroofment and drainage (n = 9), liver resection (n = 3), peritoneal lavage (n = 2), cholecystectomy (n = 4), and exploration of common bile duct (n = 2). One patient required reoperation because of bleeding. Three patients required further percutaneous drainage after surgery. The overall mortality was 46%. Four patients died of multiorgan failure and two patients died of pulmonary embolism.
CONCLUSION: Surgical treatment of pyogenic liver abscess is occasionally needed when percutaneous drainage has failed due to various reasons. Mortality rate in this group of patients has remained high.
- Citation: Ng SSM, Lee JFY, Lai PBS. Role and outcome of conventional surgery in the treatment of pyogenic liver abscess in the modern era of minimally invasive therapy. World J Gastroenterol 2008; 14(5): 747-751
- URL: https://www.wjgnet.com/1007-9327/full/v14/i5/747.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.747
When not diagnosed early and treated promptly, pyogenic liver abscess can be fatal, with reported mortality rates as high as 80%-100%[1]. Historically, the treatment of choice for pyogenic liver abscess had been open surgical drainage[2]. However, with the advent of minimally invasive therapy such as image-guided percutaneous needle aspiration or catheter drainage and the availability of broad-spectrum antibiotics, patients with pyogenic liver abscess nowadays seldom require open surgery for treatment[34]. We aim to evaluate the role and outcome of conventional surgery in the treatment of pyogenic liver abscess in the modern era of minimally invasive therapy.
Between January 1995 and December 2002, one hundred patients with pyogenic liver abscess were treated at our institution. Among them, thirteen patients required open surgical treatment. The medical records of these thirteen patients were retrospectively reviewed to determine the demographic data, clinical presentation, indications and nature of surgery, and outcome of surgery.
The diagnosis of pyogenic liver abscess was established by a combination of clinical, radiological, operative, and/or microbiological findings. All patients with suspected liver abscess were initially investigated by ultrasonography (US) and/or computed tomography (CT) to confirm the diagnosis. Additional radiological studies including endoscopic or magnetic resonance cholangiography, and barium enema were subsequently performed in selected patients to look for the underlying aetiology. Pyogenic liver abscess was considered of cryptogenic origin when no obvious extra-hepatic source of infection could be identified after appropriate investigations. After the cultures of blood and/or liver pus aspirates (either by image-guided percutaneous aspiration or open surgery) were obtained, empirical intravenous antibiotics with ampicillin 500 mg six-hourly, cefuroxime 750 mg eight-hourly, and metronidazole 500 mg eight-hourly were administered. The antibiotics therapy was adjusted according to the results of culture and sensitivity test of pus aspirates. All patients received a six-week course of antibiotics.
The exact approach of initial intervention (percutaneous or surgical treatment) was determined by the patients’ clinical condition, the certainty of diagnosis, the radiological findings, and the surgeons’ preference. Percutaneous needle aspiration or catheter drainage was done under US-guidance, the technique of which had been reported previously[5]. Patients who failed percutaneous treatment (defined as deterioration of patients’ clinical condition and/or persistence or progression of abscess on follow-up imaging studies) or developed procedure-related complications were subjected to surgery. For patients who underwent open surgical drainage, the abscess was located with the help of intraoperative US. Abscess wall was deroofed, and loculations were broken down. Large-bore catheters were placed within the abscess cavity for drainage. For patients with concurrent biliary pathologies, additional biliary tract procedures including cholecystectomy and exploration of common bile duct were performed. Liver resection was done for abscess with total destruction of a segment or section of liver parenchyma. Patients’ outcomes, including length of hospital stay, recurrence of liver abscess on follow-up imaging, complications, need for re-intervention, and operative mortality were recorded.
The patients were predominantly women (ten out of thirteen) with a mean age of 65 ± 17 years. The main presenting symptoms were abdominal pain (100%) and fever (77%). Four patients presented with peritonitis of uncertain pathology despite radiological investigations. Three patients presented with septic shock requiring admission to the intensive care unit. Eleven patients (84.6%) had coexisting diseases (Table 1).
| No. | Sex/age | Coexisting diseases | Clinical features | US/CT findings | Preoperative diagnosis | Aetiology |
| 1 | F/74 | History of jaundice | Peritonitis, hepatomegaly | 18 cm left lobe cyst | Ruptured liver cyst | Biliary (GS) |
| 2 | F/83 | HT, history of cholecystectomy | Pain, fever | 2 cm left lobe abscess | Liver abscess | Biliary (CBDS) |
| 3 | M/66 | DM | Pain, hepatomegaly | 12 cm right lobe abscess | Liver abscess | Cryptogenic |
| 4 | F/83 | HT, history of cholecystectomy and partial gastrectomy | Peritonitis, abdominal distension | (Dilated small bowel loops on abdominal X-ray) | Ischaemic bowel | Biliary (CBDS) |
| 5 | M/39 | Recent blunt liver trauma with partial hepatectomy | Pain, fever | Haematoma and abscess along liver resection margin | Liver abscess | Trauma |
| 6 | M/34 | Gunshot injury to liver with partial hepatectomy 4 months ago | Pain, fever, abscess pointing out | 5.5 cm abscess communicating with anterior abdominal wound | Liver abscess | Trauma |
| 7 | F/86 | HT, DM | Peritonitis, fever, hepatomegaly | Multiple liver cysts, largest one 18 cm in the right lobe | Ruptured liver cyst | Biliary (cystadenoma) |
| 8 | F/60 | RPC, history of cholecystectomy and HJ | Pain, septic shock | 5 cm left lobe abscess | Liver abscess | Biliary (RPC) |
| 9 | F/71 | Diverticular disease | Pain, fever | 13 cm right lobe abscess | Liver abscess | Portal (diverticulitis) |
| 10 | F/77 | HT, history of thyroidectomy for cancer | Pain, fever | 9 cm right lobe abscess | Liver abscess | Cryptogenic |
| 11 | F/60 | No | Peritonitis, septic shock | Suspected acute cholecystitis | Acute cholecystitis | Biliary (GS) |
| 12 | F/64 | No | Pain, septic shock | 4.5 cm left lobe abscess | Liver abscess | Cryptogenic |
| 13 | F/47 | DM | Pain, fever | 9 cm left lobe and 3.5 cm right lobe abscesses | Liver abscess | Portal (diverticulitis) |
The aetiologies of the liver abscess included biliary (n = 6), cryptogenic (n = 3), portal (n = 2), and trauma (n = 2). The median abscess size was 9 cm (range, 2-18 cm). Eleven patients had a positive pus culture (with Klebsiella in eight patients), while a positive blood culture was only present in the three patients with septic shock (all grew Klebsiella).
Seven patients underwent US-guided percutaneous drainage (PD) as the initial treatment. Of these, three patients (No. 2, 3, and 9) developed peritonitis secondary to peritoneal spillage of infected material. Another four patients (No. 5, 10, 12, and 13) failed to respond to PD because of multiloculation of the abscess. They all had persistent fever or progression of the abscess on follow-up US. Salvage surgical therapies, including deroofment and drainage, liver resection, peritoneal lavage, and additional biliary tract procedures, were required in these patients (Table 2). The median time interval between PD and salvage surgery was 3 d (range, 1-22 d).
| Initial intervention | Indications of surgery | No. | Details | Nature of surgery | Outcome | |
| Pyogenic liver abscess (n = 13) | Ultrasonography-guided drainage attempted (n = 7) | Complications arising from percutaneous drainage (n = 3) | 2 | Peritoneal spillage | D&D | Survive |
| 3 | Peritoneal spillage | Lavage | Survive | |||
| 9 | Peritoneal spillage | Lavage | Survive | |||
| Failed percutaneous drainage (n = 4) | 5 | Multiloculated abscess | D&D, debridement | Die of MOFS | ||
| 10 | Multiloculated abscess | D&D, chole, ECBD | Die of PE | |||
| 12 | Multiloculated abscess | L lat sec, chole | Die of MOFS | |||
| 13 | Multiloculated abscess | L lat sec, D&D | Survive | |||
| Straight laparotomy (n = 6) | Peritonitis of uncertain diagnosis (n = 4) | 1 | Ruptured liver cyst? | D&D, chole | Die of MOFS | |
| 4 | Ischaemic bowel? | D&D, ECBD | Die of MOFS | |||
| 7 | Ruptured liver cyst? | D&D | Survive | |||
| 11 | Acute cholecystitis? | D&D, chole | Die of PE | |||
| Percutaneous drainage not suitable (n = 2) | 6 | Multiloculated abscess pointing out | D&D, debridement | Survive | ||
| 8 | Multiloculated abscess in a septic patient | L lat sec | Survive |
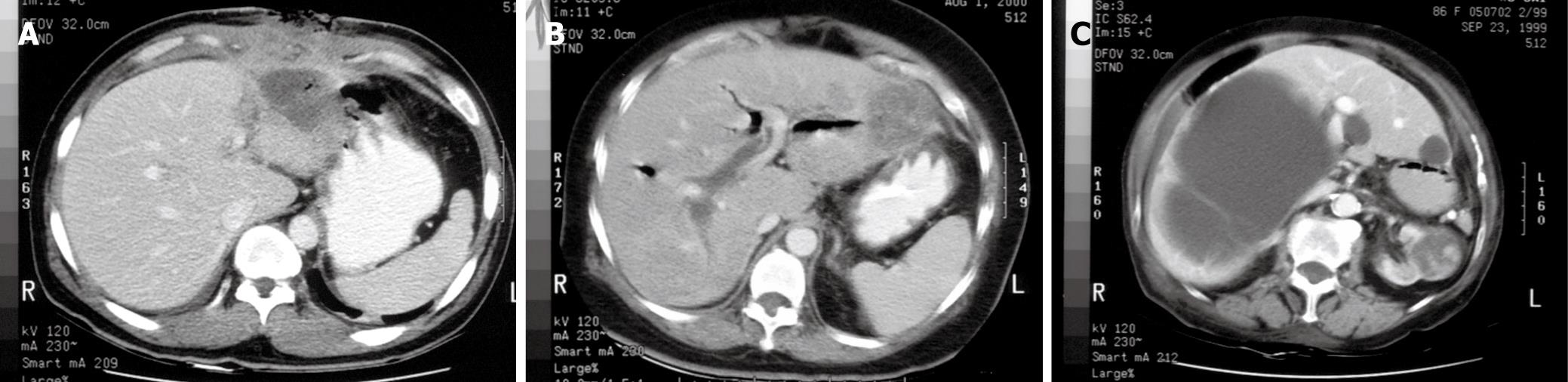
Six patients proceeded to straight laparotomy. One patient (No. 6) had undergone partial hepatectomy for gunshot injury in China four months ago. He presented with a multiloculated liver abscess that was pointing out to the anterior abdominal surface (Figure 1A). Open drainage and debridement of necrotic liver tissues was performed. Another patient (No. 8) with underlying recurrent pyogenic cholangitis and previous history of hepaticojejunostomy presented with marked sepsis from a liver abscess in the left lateral section that precluded PD (Figure 1B). She underwent left lateral sectionectomy. The remaining four patients (No. 1, 4, 7, and 11) presented with peritonitis of uncertain pathology despite radiological investigations (Figure 1C). The preoperative diagnoses were ruptured liver cysts, ischaemic bowel, and acute cholecystitis. All four patients were proven to have ruptured liver abscess on laparotomy (Table 2).
One patient (No. 1) required re-operation on day one because of the surgical complication of massive reactionary haemorrhage. Three patients required further PD after surgery because of intra-abdominal collection (No. 3 and 10) and the complication of biloma (No. 6). The overall mortality rate in this series was 46%. Four patients died of multiorgan failure and two patients died of pulmonary embolism (Table 2). For the seven patients who survived the surgery, the median hospital stay was 18 d (range, 14-27 d). None developed recurrence of liver abscess at a median follow-up of 63 mo (range, 10-108 mo).
Pyogenic liver abscess remains a rare but life-threatening disease with mortality rate as high as 80%-100% if untreated[1]. Historically, in the preantibiotic and reimaging era, the cornerstone of therapy for pyogenic liver abscess was open surgical drainage based on the classic work of Ochsner et al in 1938[2]. In 1953, McFadzean et al from Hong Kong first described the successful treatment of pyogenic liver abscess with closed aspiration and antibiotics[6]. With the introduction of high-resolution imaging modalities including US and CT in the recent two to three decades, image-guided PD has largely replaced surgical drainage as the mainstay of treatment[34]. This shift of practice has been guided by a drive for minimally invasive therapy whenever possible. PD has the advantages of avoiding general anaesthesia and an operative procedure, involves a shorter hospitalization, fewer complications, and better patient acceptance[1]. Selected series of PD report success rates of more than 90%[57]. Surgical treatment has been reserved for patients who fail to respond to PD or who have concurrent intraabdominal pathology which requires surgical management[58].
In the present retrospective study, the surgical indications of the thirteen patients with pyogenic liver abscess include failure of PD, complications arising from PD, unsuitability of PD, and uncertain diagnosis. The reason for failure of PD in our patients is due to multiloculation of the abscess. Barakate et al have identified several predictors for failure of the percutaneous approach, including abscess multiloculation, presentation with abscess rupture, biliary communication, elevated serum urea, creatinine, and total bilirubin[8]. Multiloculation contributes to poorer drainage by compartmentalization of the abscess, which reduces the effectiveness of PD. Two septic patients in our study with multiloculated abscess and concurrent intraabdominal pathology (gunshot injury of the liver and recurrent pyogenic cholangitis) who were considered to be unsuitable for PD were subjected to straight surgery. Surgical drainage allows for breakdown of loculations and more complete drainage of large multiloculated abscess. In fact, some authors have advocated surgical drainage as the first-line treatment for septic patients with multiloculated abscess, as the perceived risk of failure of PD in this group of patients is high, and any delay in adequate drainage can lead to high mortality rate[9]. Another indication for primary surgical treatment, as shown in our study, is the presentation of peritonitis of uncertain diagnosis. All four patients were proven to have ruptured liver abscess on laparotomy. According to Barakate et al, abscess rupture is the single independent risk factor predicting failure of PD, and primary surgical treatment is strongly recommended for patients with abscess rupture on presentation[8].
Biliary tract diseases have become the most common aetiology of pyogenic liver abscess in the recent decades[3478]. Although endoscopic retrograde cholangiopancreatography can easily deal with biliary obstruction due to common duct stones[10], other diseases like gallstones and recurrent pyogenic cholangitis with liver atrophy and intrahepatic duct stones will still require surgical treatment. Four patients in our study had undergone cholecystectomy for concurrent gallstone disease, while two patients had undergone exploration of common bile duct for clearance of common duct stones. Left lateral sectionectomy was performed in three patients with multiloculated abscess affecting segments II and III of the liver (one of them had underlying recurrent pyogenic cholangitis and intrahepatic duct stones and the other two had failed PD). Liver resection is particularly useful in patients with multiple abscesses or multiloculated abscess confined to a single lobe which cannot be drained even with open surgery[11].
With advancements in minimally invasive surgical techniques, laparoscopic management of pyogenic liver abscess has been shown by some authors to be feasible and safe[12–14]. The laparoscopic approach is a low-risk alternative to open surgery for patients who have failed PD[12]. As most patients requiring surgical intervention for pyogenic liver abscess are severely ill with sepsis, they may tolerate laparoscopic surgery better than open surgery. Comparing with open surgery, laparoscopic surgery has the advantages of faster recovery, less immunosuppression, shorter hospitalization, and better cosmesis[13]. Laparoscopy allows effective drainage of multiloculated abscess and thorough peritoneal lavage in case of ruptured abscess[14]. Laparoscopic US can also be done to localize concomitant abscesses and detect associated biliary pathology. In the hands of a skilled hepatobiliary and laparoscopic surgeon who is equipped with the proper tools, laparoscopic liver resection for liver abscess is also feasible[15].
The mortality rate for pyogenic liver abscess had been up to 40% until the 1980s[8]. Since then, more effective antibiotic therapy, improvement in imaging techniques, and development of image-guided PD, have resulted in published overall mortality rates between 6%-14%[5]. However, most reported series have included a heterogeneous group of patients who have undergone different treatment methods, including medical treatment alone, PD, and surgery. For the subgroup of patients undergoing surgical treatment, the mortality rate still averages 20%[16–19]. The overall mortality rate in the present surgical series is 46%. Four patients died of multiorgan failure and two patients died of pulmonary embolism. In a multivariate analysis of forty-six patients with pyogenic liver abscess, Mischinger et al found that a high Acute Physiology and Chronic Health Evaluation (APACHE) II score and the underlying disease (malignancy) but not the mode of therapy was independent predictors of mortality[18]. Chou et al also found that the systemic effects of liver abscess with sepsis and multiple organ failure were significant factors in predicting mortality[19]. In the present study, no statistical analysis of prognostic factors is performed because of the small sample size. However, we believe that the high mortality rate may be attributed to the large size of the liver abscess (median size 9 cm), a higher proportion of patients with abscess multiloculation and abscess rupture on presentation, and the associated comorbidities of the patients. Moreover, the highest mortality is observed in the subgroup of patients who failed PD and underwent salvage surgery (three out of four died). The median time interval between PD and salvage surgery was 3 d. According to Herman et al, in a septic patient with pyogenic liver abscess, aggressive therapy should be instituted as soon as possible, as any delay or failure of adequate drainage would increase morbidity and mortality[9]. For this reason, they recommended surgical drainage instead of PD as first-line treatment in septic patients, and they were able to show a low mortality of 8.5% in their series. Recently, a low mortality rate of 4.5% was also reported by Tan et al, who subjected patients with pyogenic liver abscess larger than 5 cm in size to surgical drainage as first-line treatment[20].
In conclusion, because of its minimally invasive nature, PD is still regarded as the first-line treatment for most patients with pyogenic liver abscess. Surgical treatment is occasionally required when PD has failed, when the patients have concurrent intraabdominal pathology, or when the diagnosis is uncertain. In selected group of septic patients with large and multiloculated abscess, surgical drainage may be used as the first-line treatment. However, the overall mortality rate in patients undergoing surgical treatment has remained high.
Pyogenic liver abscess remains a rare but life-threatening disease with mortality rate as high as 80%-100% if untreated. Historically, the treatment of choice for pyogenic liver abscess had been open surgical drainage. However, with the advent of minimally invasive therapy such as image-guided percutaneous needle aspiration or catheter drainage and the availability of broad-spectrum antibiotics, patients with pyogenic liver abscess nowadays seldom require open surgery for treatment. The aim of this study was to evaluate the role and outcome of conventional surgery in the treatment of pyogenic liver abscess in the modern era of minimally invasive therapy.
It is still uncertain whether first-line treatment with surgical or percutaneous drainage of large pyogenic liver abscess (size > 5 cm) results in better clinical outcome. This controversial issue can only be properly addressed by a randomised controlled trial. With advancements in minimally invasive surgical techniques, laparoscopic management of pyogenic liver abscess has been shown by some authors to be feasible and safe. Further studies to compare the efficacy and safety of laparoscopic versus open drainage of pyogenic liver abscess should be conducted.
This study does not intend to bring about any major innovations or breakthroughs in the management of pyogenic liver abscess. It mainly serves as a reminder to readers of the modern era treatment planning for pyogenic liver abscess, with a special emphasis on the role and outcome of conventional surgery in the treatment of this old but deadly pathology.
This study serves as a reminder to readers of the management algorithm of pyogenic liver abscess in the modern era of minimally invasive therapy. Because of its minimally invasive nature, percutaneous drainage is still regarded as the first-line treatment for most patients with pyogenic liver abscess. Surgical treatment is occasionally required when percutaneous drainage has failed, when the patients have concurrent intraabdominal pathology, or when the diagnosis is uncertain. In selected group of septic patients with large and multiloculated abscess, surgical drainage may be used as the first-line treatment.
‘Pyogenic liver abscess’ is a pus-filled lesion within the liver caused by bacterial infection. ‘Percutaneous drainage’ is a minimally invasive treatment option for pyogenic liver abscess, which can be done under local anaesthesia. Under X-ray or ultrasonography guidance, a fine catheter is inserted through the skin (via a tiny skin incision) into the liver abscess cavity for pus drainage.
This study serves as a reminder to readers of the modern era treatment planning for an old deadly pathology. Patients with pyogenic liver abscess are at very high risk of decompensation with rapid progression to multi-system failure and they need to be sent to appropriate centres for expedited appropriate stepwise treatment.
| 1. | Wong KP. Percutaneous drainage of pyogenic liver abscesses. World J Surg. 1990;14:492-497. |
| 2. | Ochsner A, DeBakey M, Murray S. Pyogenic abscess of the liver. II: An analysis of forty-seven cases with review of the literature. Am J Surg. 1938;40:292-319. |
| 3. | Rintoul R, O'Riordain MG, Laurenson IF, Crosbie JL, Allan PL, Garden OJ. Changing management of pyogenic liver abscess. Br J Surg. 1996;83:1215-1218. |
| 4. | Huang CJ, Pitt HA, Lipsett PA, Osterman FA Jr, Lillemoe KD, Cameron JL, Zuidema GD. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223:600-607; discussion 607-609. |
| 5. | Yu SC, Ho SS, Lau WY, Yeung DT, Yuen EH, Lee PS, Metreweli C. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932-938. |
| 6. | McFadzean AJS, Chang KPS, Wong CC. Solitary pyogenic abscess of the liver treated by closed aspiration and antibiotics: a report of 14 consecutive cases with recovery. Br J Surg. 1953;41:141-152. |
| 7. | Wong WM, Wong BC, Hui CK, Ng M, Lai KC, Tso WK, Lam SK, Lai CL. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17:1001-1007. |
| 8. | Barakate MS, Stephen MS, Waugh RC, Gallagher PJ, Solomon MJ, Storey DW, Sheldon DM. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg. 1999;69:205-209. |
| 9. | Herman P, Pugliese V, Montagnini AL, Salem MZ, Machado MA, da Cunha JE, Bacchella T, Machado MC, Pinotti HW. Pyogenic liver abscess: the role of surgical treatment. Int Surg. 1997;82:98-101. |
| 10. | Lam YH, Wong SK, Lee DW, Lau JY, Chan AC, Yiu RY, Sung JJ, Chung SS. ERCP and pyogenic liver abscess. Gastrointest Endosc. 1999;50:340-344. |
| 11. | Pitt HA. Surgical management of hepatic abscesses. World J Surg. 1990;14:498-504. |
| 12. | Yanaga K, Kitano S, Hashizume M, Ohta M, Matsumata T, Sugimachi K. Laparoscopic drainage of pyogenic liver abscess. Br J Surg. 1994;81:1022. |
| 13. | Wang W, Lee WJ, Wei PL, Chen TC, Huang MT. Laparoscopic drainage of pyogenic liver abscesses. Surg Today. 2004;34:323-325. |
| 14. | Siu WT, Chan WC, Hou SM, Li MK. Laparoscopic management of ruptured pyogenic liver abscess. Surg Laparosc Endosc. 1997;7:426-428. |
| 15. | Marks J, Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Laparoscopic liver surgery. A report on 28 patients. Surg Endosc. 1998;12:331-334. |
| 16. | Lee KT, Sheen PC, Chen JS, Ker CG. Pyogenic liver abscess: multivariate analysis of risk factors. World J Surg. 1991;15:372-376; discussion 376-377. |
| 17. | Alvarez Perez JA, Gonzalez JJ, Baldonedo RF, Sanz L, Carreno G, Junco A, Rodriguez JI, Martinez MD, Jorge JI. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181:177-186. |
| 18. | Mischinger HJ, Hauser H, Rabl H, Quehenberger F, Werkgartner G, Rubin R, Deu E. Pyogenic liver abscess: studies of therapy and analysis of risk factors. World J Surg. 1994;18:852-857; discussion 858. |
| 19. | Chou FF, Sheen-Chen SM, Chen YS, Chen MC, Chen FC, Tai DI. Prognostic factors for pyogenic abscess of the liver. J Am Coll Surg. 1994;179:727-732. |