INTRODUCTION
History of Opisthorchis viverrini
Liver flukes are platyhelminth parasites of the class trematoda, and Opisthorchis viverrini (O viverrini) is a member of the family Opisthorchiidae. Opisthorchis spp. are prevalent human parasites particularly in the Far East and South East Asia. O viverrini is highly prevalent in Thailand and Laos while C. sinensis is endemic in South China, Japan, Korea and Taiwan, O felineus is the prominent fluke in Eastern Europe[1]. O viverrini was first described in the post-mortem examination of two prisoners from a jail in Chiengmai, northern Thailand, in 1911 by Leiper[2] who obtained specimens from Kerr. Kerr[3] reported that 39 of 230 (17%) adult male prisoners examined in a prison in Chiengmai were infected with O felineus. In 1927, Prommas[4] identified the worms found at an autopsy of a 17-year-old Thai male residing in Roi-et, northeast Thailand, as O felineus. Sadun EH[5] commented that the liver fluke infection in Thailand was caused by O viverrini, not by O felineus[5], and this was confirmed later in 1965 by Wykoff et al[6].
Life cycle of O viverrini
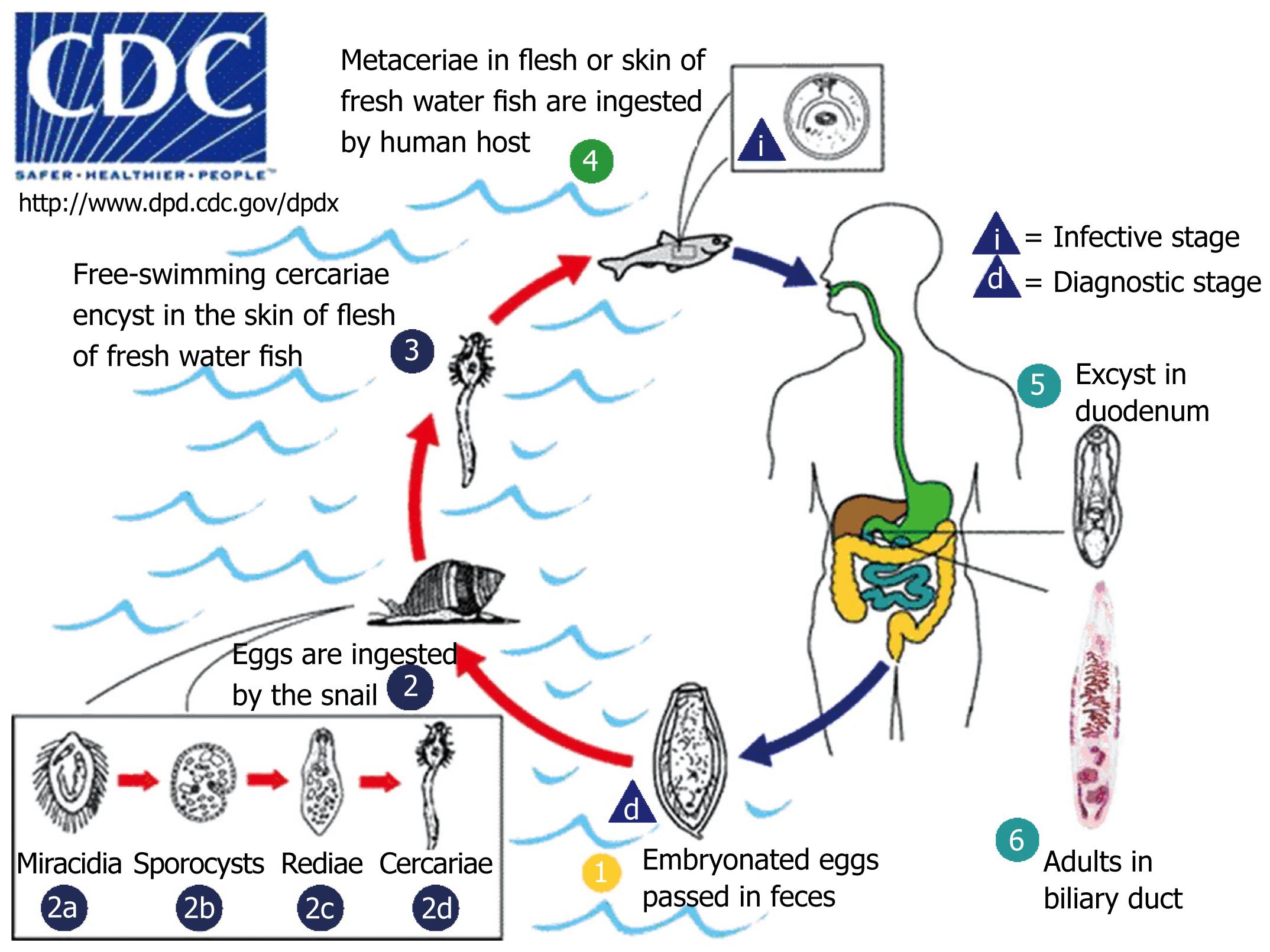
Fish-borne trematodes have a complicated life cycle with two intermediate hosts (Figure 1). Starting from a human host, the adult worms deposit fully developed eggs that are passed in the feces, the eggs from adult flukes are passed out with the faeces. The eggs must get into water in order to hatch and be able to infect their first intermediate host, a freshwater snail. After being ingested by a suitable snail, the eggs release miracidia which undergo in the snail several developmental stages: sporocysts, rediae, cercariae. The snail intermediate hosts are Bithynia goniompharus, B funiculata and B siamensis[67]. Cercariae are released from the snail and then penetrate freshwater fish that are the second intermediate host (Cyclocheilichthys spp., Puntius spp., Hampala dispa), encysting as metacercariae in the muscles or under the scales. Cats, dogs, and various fish-eating mammals including humans are the definitive host[8]. They become infected by ingesting undercooked fish containing infective metacercariae. In infected definitive host, the metacercaria excyst in the duodenum and ascend through the ampulla of Vater into the biliary ducts, where they attach and develop to adults, which lay eggs after 3 to 4 wk. The adult worms reside in the biliary system of the mammalian host, where they attach to the mucosa. The life span of O viverrini in human is not known, however, it may be over 25 years as recorded in C sinensis.
Figure 1 Life cycle of O viverrini.
Embryonated eggs are discharged in the biliary ducts and in the stool (1). Eggs are ingested by a suitable snail intermediate host (2). Each egg releases a miracidia (2a), sporocysts (2b), rediae (2c), and cercariae (2d). The cercaria is released from the snail and after a short period of free-swimming time in water, it penetrates the flesh of a freshwater fish, where it encysts as a metacercaria (3). Humans are infected through ingestion of undercooked or raw freshwater fishes (4). The metacercaria excysts in the duodenum (5) and ascends the biliary tract through the ampulla of Vater. Maturation to adulthood takes approximately one month (6). (Adapted from: http://www.dpd.cdc.gov/DPDx/HTML/Opisthorchiasis.htm).
Morphology of O viverrini
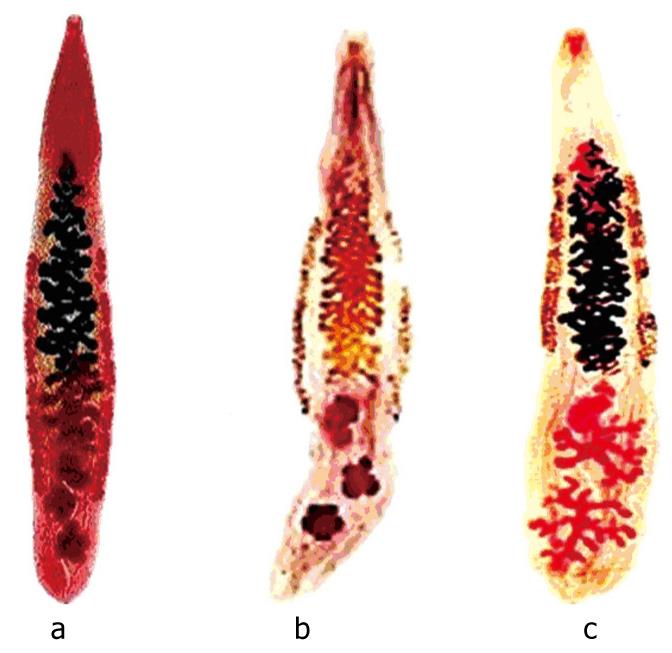
The O viverrini are dorso-ventrally flattened, lancet-shaped, thin and transparent. The adult worms are monoecious and the average size is 7.0 (5.4-10.2) × 1.5 (0.8-1.9) mm. The oral sucker is subterminal and the ventral sucker at approximately anterior one-fifth of the body length. Two testes are deeply lobed, diagonal, situated near the posterior extremity (Figure 2)[9]. O viverrini is morphologically similar to C sinensis but it is slightly smaller in size, the main difference from C sinensis. The long, slightly coiled seminal vesicle terminates in the ejaculatory duct, which opens through the genital pore immediately in front of the ventral sucker. Cirrus sac and cirrus are absent. The multilobated ovary is in front of the anterior testes and nearby is the seminal receptacle and Laurer’s canal. The vitellaria consist of numerous follicles disposed as several columnous groups in the lateral fields between ventral sucker and testes. Excretory bladder is long, sac-like tubed, S-shaped and runs between the two testes[8].
Figure 2 Adult worm of human liver flukes: a: O viverrini; b: O felineus; c: C sinensis.
The average size of O viverrini worm is 7.0 (5.4-10.2) x 1.5 (0.8-1.9) mm[8]. (Adapted from Ash, 1997).
Epidemiology of O viverrini
In Thailand, at least 15 species of cyprinoides’ fish, the freshwater fish, are able to serve as intermediate hosts of O viverini metacercariae. The tradition of eating raw or uncooked fish products is the main reason that liver fluke is a problem in Thailand. This is very popular in the northeastern and northern region, particularly in rural areas. In the northeast, three types of preparations contain uncooked, small and medium-sized, fish: (1) koi pla which is eaten soon after preparation; (2) moderately fermented pla som which is stored for a few days to weeks; and (3) pla ra which is extensively fermented, highly salted fish, and stored for at least 2-3 mo[510] (Figure 3). The consumption frequency of koi pla in the population of some communities who ate it every week was approximately 80%[11]. Studies found the highest prevalence of liver fluke infection in the northeasterners who ate koi pla[1213]. The frequencies of koi pla consumption have declined and are generally confined to special social occasions, while other under-cooked fish preparations like pla som and other moderately preserved fish are generally eaten several times a week[10]. Pla ra and jaewbhong, fully preserved fishes, are an important staple food and consumed daily by 60%-98% of northeasterners and lowland Laotians[1114]. Studies have reported that Koi pla is probably the most infective, followed by the fish preserved for < 7 d, then pla ra and jaewbhong in which viable metacercaria are rare[10].
Figure 3 Preparation of koi pla, pla som, pla ra and jaewbhong.
koi pla is prepared with the fish-based meals with local herbs, spices, condiments, rice, and vegetables. Pla som is moderately fermented or stored for a few days to weeks.Pla ra is fermented fish sauce, popular in Northeastern Thai cuisine. It is made by combining fish, salt, and roasted rice, fermenting in a pot for one to three months. jaewbhong is made by combining pla ra and chili.
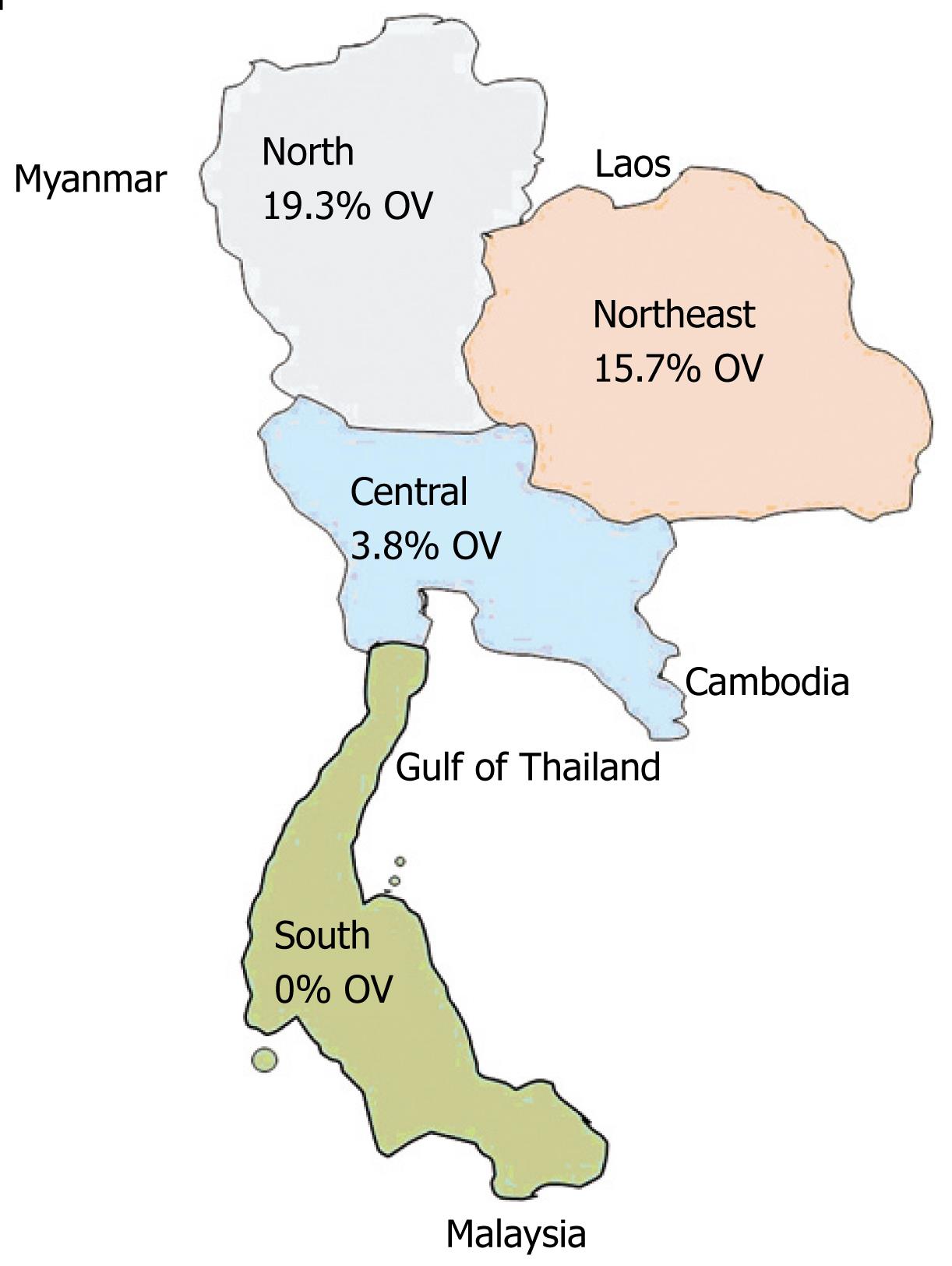
A small scale helminthiasis control program started in 1950 included the opisthorchiasis control in some high-risk areas. Following a number of studies and trial projects of the Faculty of Tropical Medicine, Mahidol University, the National Liver Fluke Control Program has been developed and operated under several National Public Health Development Plans. Presently, the program is being operated in some provinces of the Central, and all provinces of the northeast and north of Thailand[15]. The main liver fluke control strategies comprise of three interrelated approaches, namely stool examinations and treatment of positive cases with praziquantel for eliminating human host reservoir; health education for promotion of cooked fish consumption to prevent infection; and the improvement of hygienic defecation for the interruption of disease transmission[15]. The prevalences of O viverrini infection in the north (19.3%), the northeast (15.7%), the central (3.8%) and the south (0%) have recently been reported (Figure 4)[515]. The decline in the northeast from 34.6% in 1981 to current level[1617] is attributed to intensive and continuous control activities[15]. Sithithaworn et al[10] performed a cross-sectional survey of intestinal helminthes infections by examining the fecal samples collected from 332 rural northeast Thais from 3 communities during October-November 2000, and they found that the average prevalence of O viverrini infection was 14.2% (range 8.6%-19.4%)[10]. Similarly, the prevalence of O viverrini infection in Khon Kaen Province, northeast Thailand was found to be 24.5%, which was rather higher in men (27.6%) than in women (21.4%)[18]. A total of 479 stool specimens were collected from rural communities of Ubon Ratchathani Province, Thailand and examined by using two techniques: the modified Kato thick smear and the direct smear. The prevalence of O viverrini was 14.8%, being much higher than other intestinal parasite infections[19]. O viverrini is still prevalent and a serious health problem in some parts of Thailand. Therefore, the health education and promotion is still more required.
Figure 4 Prevalence of O viverrini infection in Thailand[15], OV: Percentage of O viverrini infection.
Pathology and pathogenesis of O vivierrini
Most O viverrini infection is asymptomatic. Mild symptoms include dyspepsia, abdominal pain, constipation or diarrhea. With chronic infection, the symptoms can be more severe, and hepatomegaly and malnutrition may be present. In rare cases, cholangitis, cholecystitis, and cholangiocarcinoma (CCA) may develop. The severity of the pathology appears to be associated with both intensity and duration of infection. In humans, O viverrini inhabits mainly in the intra- and extrahepatic bile ducts and, rarely, in the gall bladder and pancreatic duct. The infection can induce several pathologic changes in the liver, gall bladder and extrahepatic bile ducts. Its infection can enlarge the liver and the weight of the liver may be more than the normal up to 3000-3500 g or more in heavily infected cases. The subcapsular bile ducts of heavily infected patients are usually dilated and show prominent fibrotic wall[20–22]. Microscopically, the intrahepatic lesions of opisthorchiasis are confined to the biliary tree, particularly to the large and medium-sized bile ducts where the flukes are harboured[21]. The typical histologic changes of intrahepatic bile ducts in liver fluke infection include inflammation, epithelial desquamation, goblet cell metaplasia, epithelial and adenomatous hyperplasia and periductal fibrosis[20–23].
Periportal and periductal fibrosis appeared as the most prominent histologic feature in chronic O viverrini infection in hamster. This fibrosis correlates with the marked increase in synthesis of hepatic content of type I and, particularly, type III collagen as demonstrated in long-term O viverrini infected hamsters. Periductal inflammatory cell infiltration of lymphocytes, monocytes, macrophages, eosinophils and plasma cells was also seen both in infected hamsters[2425] and in human[21]. Granulomatous inflammation in response to the entrap parasite eggs in the periductal tissue and liver parenchyma was occasionally observed in human[26] but frequently seen in animal model[24]. Histologically, the changes seen in the gall bladder and extrahepatic bile duct in opisthorchiasis are adenomatous hyperplasia, epithelial hyperplasia and chronic inflammation[212728]. However, the studies of 189 cholecystectomy specimens in Khon Kaen revealed that fibrosis of the gall bladder wall the most common finding, among several other histopathologic features, associated with opisthorchiasis as evidenced by parasite-specific antibody. For the extrahepatic bile duct, thickened cystic duct has been noted in autopsies with opisthorchiasis. Hamsters infected with O viverrini also showed heavy inflammatory cell infiltration and periductal fibrosis[29].
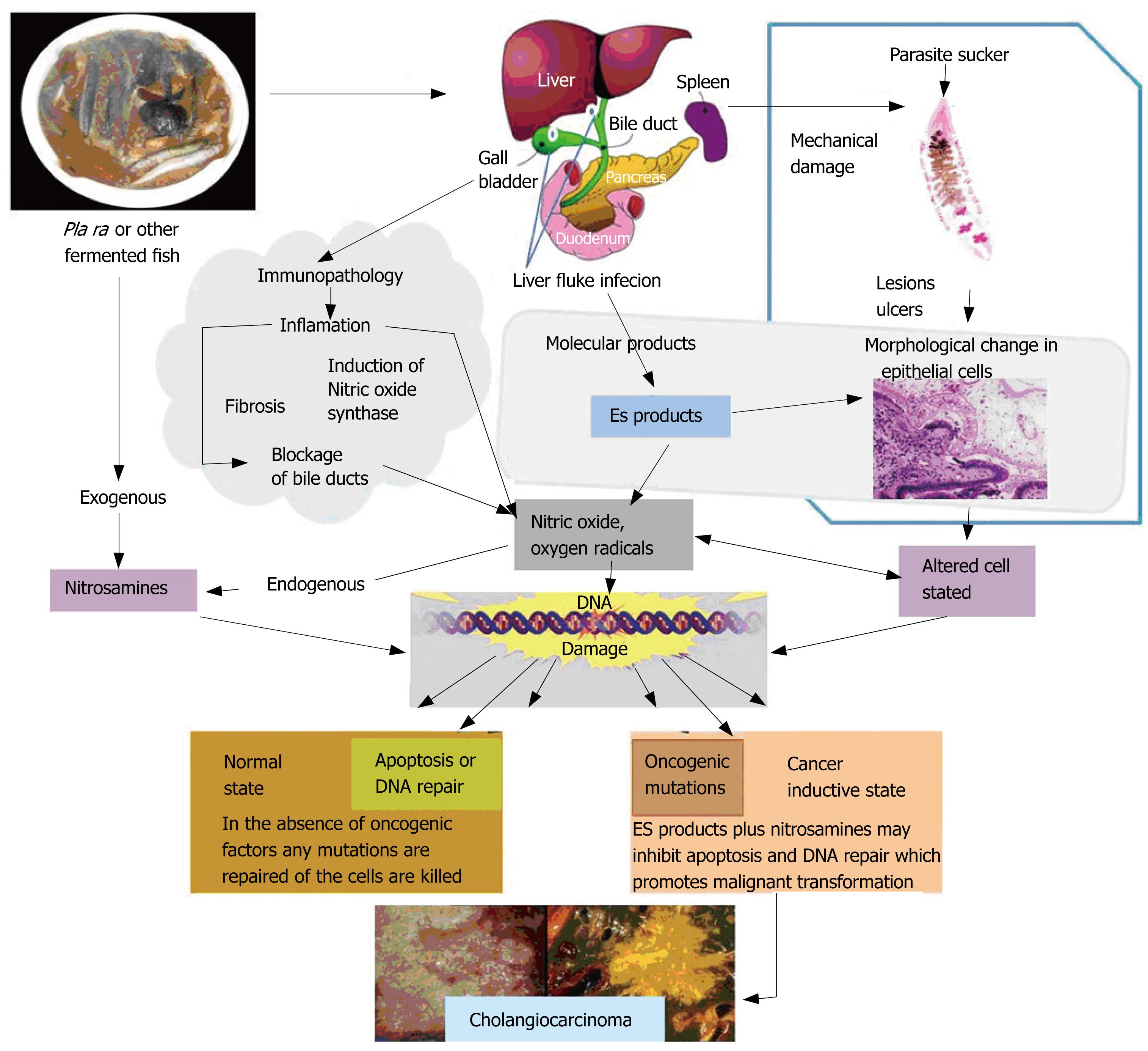
Multi-factorial etiology of CCA, mechanical damage, parasite secretions, and immunopathology may enhance cholangiocarcinogenesis (Figure 5)[3031]. The pathogenesis of O viverrini-mediated hepatobiliary changes may be due to mechanical irritation caused by the liver fluke suckers and/or its metabolic products[1824]. However, several authors suggest that immunopathological process may contribute to the hepatobiliary inflamation and damage[22242627]. Many pathological features in the hamster model reveal evidence of such mechanism, e.g. periportal infiltration of lymphocytes and macrophages. Acute damage may be induced by parasite factors, but the progressive changes are consistent with immunopathologic mechanisms[22]. During liver fluke infection, inflammation, periductal fibrosis, and proliferative responses, including epithelial hyperplasia, goblet cell metaplasia, and adenomatous hyperplasia, may represent predisposing lesions that enhance susceptibility of DNA to carcinogens[32–35]. Several N-nitroso compounds and their precursors occur at low levels in fermented food, such as preserved mud fish paste, pla ra, a condiment that is a ubiquitous component of the cuisine of northeastern Thailand and Laos[30]. Some authors hypothesised that N-nitroso compounds are a primary carcinogen leading to CCA in humans in northeastern region[3637]. Jinawath et al[38] showed the selective up-regulation of genes involved in xenobiotic metabolism in Thai patients with CCA, implying that these genes may be involved in the detoxification of possible carcinogens, such as nitrosamines. In hamster experimentally infected with O viverrini, CCA was induced by exposure of sub-carcinogenic doses of nitrosamine[34]. Liver fluke infection can induce the endogenous nitrosation both in humans and experimental animals. Infected hamster showed nitric oxide synthase (NOS) expression by immune effector cells in the inflamed areas and increased endogenous nitrosation of thiazolidine-4-carboxylic acid (thioproline)[39]. In human studies, infected cases showed a higher endogenous nitrosation than uninfected cases[4041]. Infected cases had increased levels of plasma and urinary nitrate and nitrite compared to the uninfected cases[39]. Praziquantel treatment and co-administration of ascorbic acid with proline showed enhanced immune responses to O viverrini and an increase of endogenous nitrosation[42]. Thus the aforementioned findings clearly demonstrate both exogenous and endogenous nitrosation may lead to DNA alkylation and deamination in predisposed and inflamed tissues[43–45].
Figure 5 Proposed mechanisms of Opisthorchis-derived CCA initiation.
(Adapted from Sripa et al[30]).
Molecular biology of O viverrini
Information regarding the molecular biology of O viverrini is limited in comparison of other known trematode parasites. The ribosomal DNA of O viverrini has been identified, showing the total length of approximately 13 kb consisting of 4.2 kb of large subunit rDNA, 2.0 kb of small subunit rDNA, 1.0 kb of transcribed spacer DNA and 5.8 kb of non-transcribed + external transcribed spacer DNA. The rDNA units represent 6.1% of the total genomic DNA. The small subunit gene is 1992 nucleotides long and has a G + C content of 50.94%. The use of the rRNA gene sequence comparison for determination of phylogenetic classification of O viverrini is comparable to conventional taxonomic classification[4647]. The mitochondrial cytochrome c oxidase subunit I (CO I) gene and the second internal transcribed spacer region (ITS II) gene of O viverrini were compared among O viverrini from various areas in northeast Thailand. In the nucleotide sequences of partial CO I gene (417 bp), the intraspecific variations were classified into 5 patterns but no area-specific pattern was observed. Nucleotide sequences of a region of the O viverrini ITS II gene (296 bp) from different areas were different from those of C sinensis, Haplorchis taichui, H pumilio, F gigantica, Echinostoma malayanum and Centrocestus sp[48]. DNA methylation and demethylation processes are thought be taken place in O viverrini. The O viverrini genome contains a highly repeated DNA element which is very specific to the organism[49]. The construction of DNA probe to detect genomic DNA of the parasite, for diagnosis of suspected patients or identification of parasites in suspected specimens from endemic areas was developed. The construction of specific DNA probe O viverrini was achieved using a unit of highly repeated DNA element (334 base pairs)[50]. The specific DNA probe was characterized and used for detecting the presence of eggs DNA in stool samples[5152]. Specific primers were designed and used for PCR-based detection. The PCR detection was highly specific and 100% positive. Further studies on molecular biology of O viverrini are needed to better understanding of the parasite and host-trematode relationship. Several other molecular studies of O viverrini have been reported, including cloning and expression of glutathione S-transferase genes[5354].
Laha et al[55] reported the gene discovery effort for O viverrini that should expedite molecular studies of cholangiocarcinogenesis and accelerate research focused on developing new interventions, drugs and vaccines, to control O viverrini and related flukes. Approximately 5000 randomly selected cDNAs from the adult stage of O viverrini were characterized and accounted for 1932 contigs, representing about 14% of the entire transcriptome, and, presently, the largest sequence dataset for any species of liver fluke. Twenty percent of contigs were assigned GO classifications. Abundantly represented protein families included those involved in physiological functions that are essential to parasitism, such as anaerobic respiration, reproduction, detoxification, surface maintenance and feeding. A total of 164 O viverrini contigs contained ORFs with signal sequences, many of which were platyhelminth-specific, such as convergent evolution between host and parasite secreted/membrane proteins identified were homologues of vaccine antigens from other helminths. Finally, ORFs representing secreted proteins with known roles in tumorigenesis were identified, and these might play roles in the pathogenesis of O viverrini-induced CCA[55]. One of candidate gene, cysteine protease was studied by Kaewpitoon et al (unpublished data). By using specific fluorogenic substrates, majority of O viverrini protease was cysteine protease cathepsin L whereas serine and aspartic protease showed very low activities. Molecular cloning of cysteine protease encoding gene by RACE-PCR using degenerated oligonucleotide primers derived from conserved active site obtained a full-length mRNA of 981 bp encoding for 326 amino acids (GenBank, accession number AY821800). The cDNA of the novel O viverrini cysteine protease was designated Ov-cp-1. Sequence analysis showed high homologies with cysteine proteases of other trematodes, such as Clonorchis sinensis (85%) and Paragonimus westermani (55%). Ov-cp-1 cDNA expression was observed in various developmental stages of O viverrini including the metacercariae, juvenile (1, 2, 3 wk), adult worms, and eggs. Polyclonal antibody to Ov-CP-1, expressed protein, showed strong reaction with the gut epithelium, testes, vitelline glands and eggs in the uterus of the adult worm. Further functional study revealed that Ov-CP-1 could digest human hemoglobin and the activity was inhibited by the addition of E-64. The results suggest that the recombinant Opisthorchis cysteine protease Ov-CP-1 is potential for development of host-parasite relationship.
Immunity to O viverrini infection
O viverrini infection can induce both cellular and humoral host immune responses. Humoral immune response has been reported in both humans and animal model. The parasite antigens were detected in the epithelial lining of intrahepatic and extrahepatic bile ducts as early as d 3 after hamsters were infected with O viverrini and stimulated the host immune system[25]. The antibody levels in the patients with severe opisthorchiasis were significantly higher than those with mild infection. The infected hamster serum contained a component, possibly specific immunoglobulin(s) capable of reacting with adult, juvenile, excretory-secretory (Es) and egg antigens[56–61]. Antibody response to both somatic and Es antigens were detected[6062]. The antibody responses in hamsters were first detected as early as 7-14 d post-infection. During early infection, hamsters infected with 50 or 100 metacercariae (Mc) showed higher antibody levels than those infected with 25 Mc. Then, the antibody level increased rapidly to a plateau at approximately 2 mo. The antibody levels to egg and somatic, but not to Es antigens, were significantly higher in the hamsters infected with 25 Mc than in those infected with 50 or 100 Mc. These antibody responses, particularly to egg and Es antigens, did not correlate with worm burden or egg output[59]. Secretory IgA antibody reacted with both egg and Es antigens also appeared in the bile of infected hamsters. IgG, IgA and IgE antibodies in serum and bile have been reported in patients with opisthorchiasis[61–64]. Analysis of bile and serum from individual patients showed that while IgG was the predominant in the serum of all patients, IgA was present at approximately the same level as IgG or higher in the bile, while IgE antibody could be detected in 50% of the bile samples[64]. The IgG levels to the somatic antigen were most markedly elevated in disease cases and were closely associated with gall bladder size and dysfunction, and also significantly correlated with opisthorchis egg counts[32]. The antibody response to O viverrini decreased after treatment[6566]. Immunoblot analysis revealed a 91/93-ku metacercaria homogenate doublet which was recognized only in sera of individuals with severe liver disease on ultrasound findings, including cholangiocarcinoma[646768].
Cell-mediated immune responses (CMIR) to O viverrini infection have been demonstrated. Granulomatous inflamatory response to adult flukes and eggs during chronic infection is one of the typical CMIR. The inflammatory granuloma is surrounded by eosinophilic precipitates, neutrophils, eosinophils, mononuclear cells and macrophages after 6 wk of infection[24]. Similarly, patients infected with C sinensis exhibited egg-induced granuloma, both in the gall bladder and in the liver portal area[6970]. Although both cellular and humoral immunity to O viverrini have been reported, there exists a striking difference in the antigenic recognition pattern of T and B cells which may be important for selecting antigen molecules for immunological studies of this trematode infection in man[71]. The molecular weight of the main immunogenic fractions for T cells ranged from 28 to 46 ku, whereas those recognized by antibodies were 45, 52, 56, 59, 65, 69, 75 and 81 ku. The exposure to O viverrini depressed the egg and egg reduction could be due to biliary obstruction and not necessarily to immunological damage of the reproductive system of the flukes. Biliary obstruction is unlikely to be the major factor as it is known to be uncommon in experimental opisthorchiasis[2472]. Infection of hamsters with O viverrini, whether or not eliminated with praziquantel before the time of the re-challenge, failed to confer any protective immunity to reinfection[73].
An attempt to potentiate acquired immunity in hamsters with prior infection by immunizing them with aqueous somatic extract of adult worms resulted in a 30% reduction of worm[74]. IgG, IgA and IgM were found significantly higher levels to adult worm homogenate and metacercaria homogenate in stool of the egg-negative residents in the O viverrini-endemic area comparing to stool of the egg-positive group, thereby indicating some protective evidence of opisthorchiasis[75]. The lack of protective immunity is perhaps due to suppression of both cellular and humoral immune responses to unrelated antigens by O viverrini as shown by reduced phytohemagglutinin-induced lymphoproliferation and response to sheep red blood cell stimulation[6875]. Moreover, when specific antibody was measured in the infected animals, the titer was found to be depressed during the late stage of infection, particularly in heavily infected group[77]. The parasites may be resistant to immune damage or able to evade the defense system of the host. The inability to kill the worms by serum from infected animals or from patients with opisthorchiasis, and the resistance to immune damage may be related to tegumental shedding and repair[6876]. The role of T cells and cytokines in protective immunity and pathogenesis of opisthorchiasis is also unclear. The set of cytokines produced by these different T helper responses, in turn, can exert opposing effects on the parasite, resulting in either control of infection or promotion of disease. O viverrini may induce the Th2 response as found with other flukes, such as Schistosoma mansoni. However, information regarding the significance of immune responses generated during liver fluke infections is limited[68].
Diagnosis of O viverrini infection
Diagnosis of the infection is basically based on the standard fecal examination of the fluke’s eggs. However, in cases with bile duct obstruction or in a light infection, eggs may not be detected in the feces[77]. Detection of fecal Opisthorchis antigen has been reported[7879], though it is not so convenient. There is a need for a reliable diagnostic tool to solve the clinical and parasitological diagnosis problems in opisthorchiasis. Immunodiagnostic method is one of highly sensitive techniques in diagnosis of O viverrini infection. Several serologic tests for the diagnosis of opisthorchiasis have been reported[586578–83]. However, some cross-reactions with other parasitic infection are still a problem. The recombinant cysteine proteinase-based ELISA was used to diagnose the infected hamsters and humans. The use of Ov-CP-1 as antigens showed that humoral immune responses in hamsters infected with 50 Metaceriae of O viverrini occured as early as d 15 post-infection and rapidly increased at one month before reaching a plateau at mo 2, which is in agreement with that reported by using O viverrini-somatic extract, excretory-secretory product or egg antigens[58]. In human cases, the Ov-CP-1-based ELISA did not cross-react with sera from patients with hookworm, minute intestinal fluke, S stercoralis, Taenia sp., G lamblia and E. coli infection. The sensitivity and specificity of the test were 94.66% and 95.55%, respectively. This is even higher than conventional indirect ELISA using O viverrini somatic extracts or excretory-secretory product antigen[63647981]. The sensitivity and specificity are similar to other studies using ELISA based on recombinant trematode cysteine protease, such as C sinensis (sensitivity: 81.3%-96%; specificity: 92.6%-96.2%)[8485] and P. westermani (sensitivity: 86.2%-93%; specificity: 93%-98%)[8687]. It seems that the more specific purified antigen used, the more sensitivity and specificity obtained. Recently, Ruangsittichai et al[54] reported a high sensitivity and specificity of a recombinant eggshell protein (ESP) as a potential serodiagnosis of human opisthorchiasis. However, detection of O viverrini DNA is expensive and requires skillful professional personnel. This overall result suggests that Ov-CP-1 has potential as an antigen for ELISA-based immunodiagnosis of opisthorchiasis.
PCR-based amplification of parasite DNA from stools has been documented for its high specificity. Compared with that of formalin-ether and Stoll's egg count, the sensitivity of the PCR was found to be 100%, 68.2%, and 50% in the fecal specimens containing > 1000, 200-1000, and < 200 eggs per gram of feces, respectively. Human fecal materials showed a strong reduction in PCR sensitivity of detection[5152]. Recently, an adult stage O viverrini cDNA library was constructed and was isolated a glutathione S-transferase (GST) cDNA. The cDNA has a size of 893 bp and encodes a GST of 213 amino acids (OV28GST). The OV28GST RNA was detected in the parenchymal cells of adult parasites. Both purified recombinant and purified native OV28GST were resolved as 28-ku proteins. Molecular analysis of OV28GST is helpful in ongoing development of diagnostic applications for O viverrini[54].
O viverrini is a medically important food-borne trematode in some part of Southeast Asian countries, including Thailand. The opisthorchiasis has been studied for more than 50 years, a major break through in pathology and pathogenesis of opisthorchiasis was the discovery of Opisthorchis antigens in the biliary epithelium and its associated intense inflammatory response in certain infected animals[25]. The infection is associated with cholangiocarcinoma and other hepatobiliary diseases. The epidemiology is found in some rural areas because of the tradition of eating raw or uncooked fish products. The health education programs to prevent and control opisthorchiasis are still required in the high-risk areas.