Published online Dec 21, 2008. doi: 10.3748/wjg.14.7208
Revised: October 13, 2008
Accepted: October 20, 2008
Published online: December 21, 2008
AIM: To evaluate the safety and efficacy of vitamin E in children with chronic hepatitis B.
METHODS: We randomly assigned patients with chronic hepatitis B, positive for hepatitis B e antigen (HBeAg), to receive either vitamin E or placebo once daily for 6 mo in a 3:1 ratio and double-blind manner. The primary end point was HBeAg seroconversion, defined as the loss of HBeAg, undetectable levels of serum hepatitis B virus DNA, and the appearance of antibodies against HBeAg 12 mo after therapy.
RESULTS: At baseline visit, 49 patients had normal and 43 had increased serum aminotransferase levels. Twenty-nine patients did not respond to previous treatment with interferon-α or lamivudine. Seventy-six children completed the study; 16 were non-compliant (n = 7), lost to follow-up (n = 7), or started another antiviral treatment (n = 3). Intention-to-treat analysis showed HBeAg seroconversion in 16 children (23.2%) treated with vitamin E and two (8.7%) in the placebo group (P = 0.13). Vitamin E was well tolerated.
CONCLUSION: There is only a tendency that vitamin E may promote HBeAg seroconversion. Therefore larger studies are needed to clarify the role of antioxidants in the therapy of chronic hepatitis B.
- Citation: Gerner P, Posselt HG, Krahl A, Ballauff A, Innerhofer A, Binder C, Wenzl TG, Zense M, Hector A, Dockter G, Adam R, Neubert J, Claßen M, Gemmern RV, Wirth S. Vitamin E treatment for children with chronic hepatitis B: A randomized placebo controlled trial. World J Gastroenterol 2008; 14(47): 7208-7213
- URL: https://www.wjgnet.com/1007-9327/full/v14/i47/7208.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7208
| Vitamin E | Placebo | |
| Total | 56 | 20 |
| Sex (%) | ||
| Female | 22 (39.3) | 8 (40) |
| Male | 34 (60.7) | 12 (60) |
| Age (yr) | 10.4 | 11.8 |
| Alanine transaminase level (%) | ||
| Normal | 33 (58.9) | 13 (65) |
| Elevated | 23 (41.1) | 7 (35) |
| More than double above normal level | 8 | 2 |
| HBV-DNA (%) | ||
| < 1000 pg/mL | 22 (39.3) | 7 (35) |
| > 1000 pg/mL | 35 (60.7) | 13 (65) |
| Route of transmission (%) | ||
| Parenteral | 1 (1.8) | 1 (5) |
| Vertical | 34 (60.7) | 10 (50) |
| Unknown | 21 (37.5) | 9 (45) |
| Interferon alpha pre-treatment (%)1 | 11 (19.6) | 4 (20) |
| Lamivudine pre-treatment (%)1 | 5 (8.9) | 3 (15) |
| HBeAg seroconversion (%)2 | 16 (28.6) | 2 (10) |
| Vitamin E | Placebo | |
| Total | 16 | 2 |
| Sex (%) | ||
| Female | 10 (62.5) | 2 (100) |
| Male | 6 (37.5) | 0 |
| Age (yr) | 12.4 | 14.2 |
| Alanine transaminase level (%) | ||
| Normal | 8 (50) | 2 (100) |
| Elevated | 8 (50) | 0 |
| HBV-DNA (%) | ||
| < 1000 pg/mL | 12 (75) | 1 (50) |
| > 1000 pg/mL | 4 (25) | 1 (50) |
| Route of transmission (%) | ||
| Parenteral | 1 (6.3) | 0 |
| Vertical | 8 (50) | 0 |
| Unknown | 7 (43.7) | 2 (50) |
| Interferon alpha pretreatment (%) | 2 (11) | 0 |
| Lamivudine pretreatment (%) | 0 | 1 (50) |
| Dosage (%) | ||
| 200 IU Vitamin E | 0 | |
| 400 IU Vitamin E | 4 (25) | |
| 600 IU Vitamin E | 12 (75) | |
| HBeAg seroconversion after initiation of treatment | ||
| 6 mo | 6 | 0 |
| 12 mo | 7 | 1 |
| 18 mo | 3 | 1 |
More than 350 million people worldwide are chronically infected with hepatitis B virus (HBV). Chronic HBV infection causes cirrhosis, hepatocellular carcinoma, and end-stage liver disease which accounts for approximately 1 million deaths each year. In most countries horizontal transmission of HBV is the main route of infection during childhood[1-3]. In this age group the first stage of infection is characterized by the presence of hepatitis B s antigen (HBsAg) and hepatitis B e antigen (HBeAg) in serum, a high virus load, and low inflammatory activity. These parameters indicate a low or absent immune response against HBV which can persist for decades. The great majority of patients in this “immune tolerance” phase have no sustained response to current treatment[4]. However, it is known that chronic HBV carriers show spontaneous HBeAg seroconversion at approximately 10% per year[5]. Among patients with predictors of beneficial response such as high levels of serum transaminases and low levels of HBV DNA, only approximately one-third respond to interferon alpha[6-9] and about 20% respond to nucleoside analogues[10-12]. Furthermore, it has been shown that interferon alpha treatment simply accelerates anti-HBe seroconversion[13]. The question of whether it is worth treating a patient is further complicated by the significant side effects of interferon alpha treatment and the potential to develop resistance to nucleoside analogues. Therefore further treatment options are needed.
Since a defective immune response is likely to be one factor in the pathogenesis of liver damage caused by HBV, immunomodulatory substances have been tested for the treatment of chronic hepatitis B[14-19] but the results are conflicting. In a recent placebo controlled study, Andreone et al[20] tested vitamin E for chronic hepatitis B. In this pilot trial, the response rate was significantly higher in patients in the vitamin E group than in the placebo group.
Vitamin E is an antioxidant in the cell membrane which acts as a scavenger of free radicals. In patients suffering from various hepatopathies, it is able to protect against liver damage[21,22]. Vitamin E was also shown to enhance clinically relevant T-cell-mediated function[23].
The purpose of this study was to evaluate the safety and efficacy of a high-dose 6-mo course of vitamin E in a large number of chronically infected children. Our study included a 12-mo follow-up period, which allowed us to evaluate post-treatment effects and the durability of response.
We conducted a prospective, randomized, double-blind, placebo-controlled study of patients with chronic hepatitis B in 22 German centers and one Austrian center. The dose of vitamin E depended on the patients’ weight. Children below 20 kg received 200 IU of RRR-α-tocopheryl acetate concentrate once daily for 6 mo. Patients between 20 and 40 kg received 400 IU and patients of more than 40 kg received 600 IU once daily for 6 mo (Cognis, Nutrition&health, Düsseldorf, Germany). After the treatment period, patients were followed up for 12 mo. Placebo capsules were indistinguishable from vitamin E capsules and were produced by the same company. Eligible patients were randomized to receive either vitamin E or placebo in a 3:1 ratio. Patients were randomly assigned by a computer-generated program at the study center (Children’s Hospital, Helios Klinikum Wuppertal, Germany).
The local ethics committee (Witten-Herdecke University, Germany) approved this study. Written consent was given from parents and also from the patient if older than 11 years.
Inclusion criteria were: (1) age between 1 and 17 years; (2) presence of HBsAg and HBeAg for at least 6 mo; (3) presence of HBV DNA in serum for at least 6 mo (HBV-DNA > 10 000 copies/mL).
Exclusion criteria were: (1) antiviral therapy within the 6 mo prior to the study; (2) concurrent participation in another clinical trial; (3) hepatic decompensation; (4) co-infection with hepatitis C, hepatitis D or human immunodeficiency virus; (5) pregnancy or breast feeding.
The following biochemical and virological data were examined at baseline visit and 2, 4, 6, 12 and 18 mo after starting therapy: vitamin E, aspartate aminotransferases, γ-glutamyl transferase, complete blood count, thromboplastin time, thyroid stimulating antibodies, HBV DNA, HBeAg, anti-HBe, HBsAg, anti-HBs. At each visit, the child underwent a physical examination and was interviewed regarding potential side effects and adverse reactions. Biochemical and serological markers of hepatitis B infection were tested in each center.
The primary end point for efficacy was seroconversion to anti-HBe, loss of HBeAg and loss of HBV DNA at the end of follow-up (12 mo after treatment), defined as HBV-DNA level under 400 copies/mL or in other tests < 5 pg/mL.
The comparison of groups with respect to responses at 18 mo was done by Fisher’s exact test.
Ninety-two children and adolescents were enrolled in the study. Baseline characteristics of the patients are shown in Table 1. Vitamin E and placebo groups were similar with respect to demographic and clinical characteristics. Of the 76 patients completing the study, 18 (16 vitamin E and two placebo) responded to therapy as defined by HBeAg seroconversion, loss of HBeAg and HBV DNA (< 400 copies/mL) and normalization of alanine aminotransferase (ALT).
Of the 92 patients enrolled, 16 were excluded (13 of the vitamin E and three of the placebo group). Seven patients were non-compliant as medication was taken only for a reduced period or was taken irregularly and one patient administered additional self-medication with vitamin E. Seven patients were lost to follow-up and three patients started treatment with interferon alpha or lamivudine.
Twenty-six of the 69 (37.7%) patients in the vitamin E group received 600 IU, 30 (43.5%) 400 IU and eight (11.6%) 200 IU.
Patients were classified into two groups: one with normal transaminase levels and the second with elevated transaminase levels. In the children who responded to therapy, eight patients had normal transaminase levels before treatment and eight patients had elevated transaminases, while all children with response in the placebo group had elevated transaminases (Table 2). Eighteen children seroconverted to anti-HBe and 15 of them showed ALT elevation before seroconversion (mean 3.2 times normal range). In three of these patients transaminases increased slightly only before seroconversion while the rest of them had a mild transaminase flare immediately before and during anti-HBe seroconversion. None of them showed acute exacerbation of hepatitis B.
Patients were classified into two groups: one with low HBV DNA titers of less than 1000 pg/mL and one with high HBV DNA of more than 1000 pg/mL. Twelve (75%) patients with low and four (25%) with high viral DNA responded to vitamin E. In the two responders in the placebo group, one patient had high HBV DNA titers and one low.
Thirty-four patients (49.3%) in the vitamin E group and 10 patients (43.5%) in the placebo group were likely to be infected by her mother, either perinatally or later in childhood. Of these, nine children responded to vitamin E treatment; none of the children in the placebo group responded. The route of infection was unknown in 21 children (30.4%) in the vitamin E group and nine (39.1%) in the placebo group. Eight and two children responded to therapy, respectively. The only patient with parenteral infection in the vitamin E group responded to therapy while the child in the placebo group did not.
The therapy was well tolerated. Three children treated with vitamin E experienced self-limited gastroenteritis of 1-3 wk duration. Monitoring of the above-mentioned biochemical and clinical data did not reveal any other side effects or adverse events. No child had before or during follow-up abnormal TSH or thromboplastin time. There was no significant increase in the GGT-level and no pathological change of the whole blood count.
In the vitamin E group, six patients responded during vitamin E treatment, seven within the first year and three at 18 mo after the start of therapy. In the placebo group, both patients responded only during the follow-up period at 12 and 18 mo. During anti-HBe seroconversion and loss of HBeAg no child developed acute exacerbation of hepatitis B.
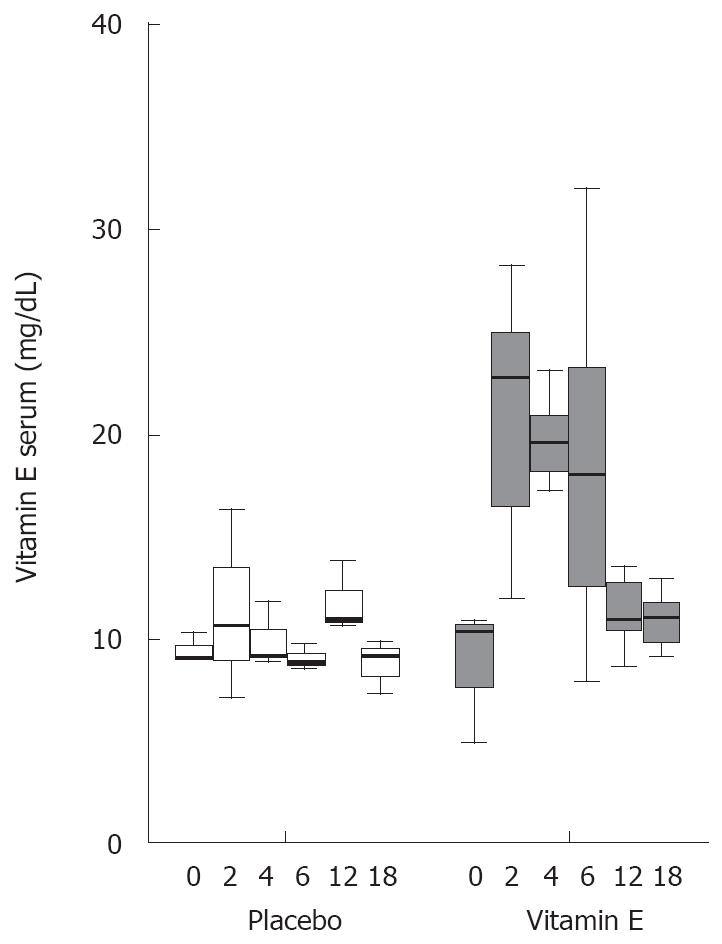
A significant increase in vitamin E levels was observed in the vitamin E group. Vitamin E levels doubled within 2 mo and fell to starting levels immediately after cessation of therapy. No significant change in vitamin E levels was found in the placebo group (Figure 1).
The results of this study suggest that vitamin E is well tolerated in children and adolescents with chronic hepatitis B and yields a higher HBeAg seroconversion rate than patients treated with placebo. Among 69 children treated with vitamin E, 23.2% became negative for serum markers of viral replication (HBeAg and HBV DNA). As expected, an 8.7% spontaneous rate of anti-HBe seroconversion and loss of HBeAg was found in the control group, in line with previous reports[5,8,24-26]. The response rate in vitamin E-treated-children was slightly worse than in patients treated with interferon alpha[5,8,24-26] and comparable with the treatment with nucleoside analogues[10-12]. This is surprising since the majority of children treated in our study had poor predictors of virological response. Among children who completed the study, approximately two-thirds had normal ALT levels and high levels of viremia, and one-third were resistant to one or two previous antiviral treatments. In the study of Andreone et al[20], 7 of 15 patients treated with vitamin E showed normalization of ALT levels, 8 of 15 became negative for HBV DNA, and 3 of 7 HBeAg positive patients achieved seroconversion which differed significantly from the placebo group. It is known that seroconversion of HBeAg may occur after years of treatment with interferon or lamivudine. In our study population, 19 children were treated with interferon or lamivudine before treatment with vitamin E (Table 1). Only one child was treated 7-12 mo before entering this study with interferon, and another three patients were treated 12-36 mo before starting this study. The remaining patients received antiviral therapy more than 2 years before the study. We therefore think that antiviral therapy prior to the study should not be a significant bias of the results of this study.
It is known that vitamin E improves the amino-transferase status in patients with various liver diseases such as hepatitis C, hemochromatosis and Wilson’s disease[21,22]. This effect is probably based on its antioxidant properties, resulting in the protection of liver damage by oxidative stress. However, immunomodulatory effects are also documented. It was shown in vivo that vitamin E enhances T cell-mediated function, acts as a proliferative stimulator of lymphocytes and has natural killer cell activity[23,27,28]. Furthermore, T helper cells are polarized towards the T helper-1 phenotype which is known to be under-represented in chronic hepatitis B carriers[29]. It can be hypothesized that these effects of vitamin E may be a factor in the antiviral immune response seen in our patients.
According to current consensus statements on the treatment of HBeAg positive hepatitis B, only patients with ALT levels greater than double normal values, or moderate/severe hepatitis on biopsy should be considered for therapy[30-32]. In childhood, however, the majority of patients are immune tolerant to HBV, resulting in no significant liver inflammation. The results of this study suggest that vitamin E may be used for these patients; this is supported by the finding that half of the patients with HBeAg seroconversion had normal aminotransferases before treatment. Moreover, it may be useful for patients who have previously failed with interferon alpha or nucleoside analogues as re-treatment with the same drugs either as monotherapy or in combination[33,34]. The excellent safety of vitamin E found in this study and in many other reports, even in long-term treatment, further favors this therapy[35].
Although the HBeAg seroconversion rate was higher in the vitamin E group, our study population is too small to show a significant difference between the two groups. Our study is hampered by the relatively high number of patients who had to be excluded because of non-compliance or loss of follow-up. This may in part be due to the long follow-up period and the high number of treatment centers involved in this study. Furthermore, some patients may not have been convinced that vitamin E can be used as a “real” drug and can be effective against hepatitis B. The results of this study may, however, improve the motivation of such patients and lead to a bigger study population.
We conclude that treatment with high doses of vitamin E is well tolerated and may promote HBeAg seroconversion even in difficult to treat patients, but further studies are warranted to verify the effectiveness of vitamin E.
With an anual mortality of around 800 000 patients per year, chronic hepatitis B is one of the most serious worldwide health problems.
Chronic hepatitis B can be regarded as a difficult to treat disease. Despite treatment options like interferon-alpha and nucleos(t)ide analogues, the majority of patients fail to respond to treatment. Moreover during the so-called immune tolerant phase of HBV infection, no treatment can be offered to the patient as the chance to respond to treatment is minimal.
Recent reports have demonstrated that treatment with vitamin E can normalize liver transaminases and may also lead to HBeAg seroconversion. In this report, studied for the first time in children, the effect of vitamin E was evaluated in a placebo-controlled trial. The overall effect in the vitamin E group was higher than in the placebo group but this effect did not reach significance.
In particular, children are often in the immune tolerant phase of infection and therefore no treatement is indicated. As vitamin E has an excellent side-effect profile, it may be used in otherwise not treatable children. This has however been evaluated in larger studies.
Authors investigated the putative role of vitamin E in chronic hepatitis B. The study was conducted carefully and the double-blinded placebo-controlled trial reaches a high standard.
Peer reviewer: Seyed-Moayed Alavian, Associate Professor of Gastroenterology and Hepatology, Department of Internal Medicine, Baqiyatallah University of Medical Sciences & Tehran Hepatitis Center, PO Box 14155-3651-Tehran, Iran
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38 Suppl 2:S5-S12. |
| 2. | Hahne S, Ramsay M, Balogun K, Edmunds WJ, Mortimer P. Incidence and routes of transmission of hepatitis B virus in England and Wales, 1995-2000: implications for immunisation policy. J Clin Virol. 2004;29:211-220. |
| 3. | Yao GB. Importance of perinatal versus horizontal transmission of hepatitis B virus infection in China. Gut. 1996;38 Suppl 2:S39-S42. |
| 4. | Lai CL, Lok AS, Lin HJ, Wu PC, Yeoh EK, Yeung CY. Placebo-controlled trial of recombinant alpha 2-interferon in Chinese HBsAg-carrier children. Lancet. 1987;2:877-880. |
| 5. | Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, Lai ST, Wong WM, Lai LS, Poon RT. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690-698. |
| 6. | Korenman J, Baker B, Waggoner J, Everhart JE, Di Bisceglie AM, Hoofnagle JH. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991;114:629-634. |
| 7. | Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Haussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422-1427. |
| 8. | Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, Rosenthal P, Lachaux A, Shelton M, Sarles J. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988-995. |
| 9. | van Zonneveld M, Honkoop P, Hansen BE, Niesters HG, Murad SD, de Man RA, Schalm SW, Janssen HL. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology. 2004;39:804-810. |
| 10. | Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263. |
| 11. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. |
| 12. | Jonas MM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, Greensmith MJ, Gardner SD, Bell MS, Sokal EM. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002;346:1706-1713. |
| 13. | Bortolotti F, Jara P, Barbera C, Gregorio GV, Vegnente A, Zancan L, Hierro L, Crivellaro C, Vergani GM, Iorio R. Long term effect of alpha interferon in children with chronic hepatitis B. Gut. 2000;46:715-718. |
| 14. | Fattovich G, Brollo L, Pontisso P, Pornaro E, Rugge M, Alberti A, Realdi G. Levamisole therapy in chronic type B hepatitis. Results of a double-blind randomized trial. Gastroenterology. 1986;91:692-696. |
| 15. | Fattovich G, Giustina G, Alberti A, Guido M, Pontisso P, Favarato S, Benvegno L, Ruol A. A randomized controlled trial of thymopentin therapy in patients with chronic hepatitis B. J Hepatol. 1994;21:361-366. |
| 16. | Mutchnick MG, Lindsay KL, Schiff ER, Cummings GD, Appelman HD, Peleman RR, Silva M, Roach KC, Simmons F, Milstein S. Thymosin alpha1 treatment of chronic hepatitis B: results of a phase III multicentre, randomized, double-blind and placebo-controlled study. J Viral Hepat. 1999;6:397-403. |
| 17. | Farhat BA, Marinos G, Daniels HM, Naoumov NV, Williams R. Evaluation of efficacy and safety of thymus humoral factor-gamma 2 in the management of chronic hepatitis B. J Hepatol. 1995;23:21-27. |
| 18. | Arase Y, Tsubota A, Suzuki Y, Suzuki F, Kobayashi M, Someya T, Akuta N, Hosaka T, Saitoh S, Ikeda K. A pilot study of thymosin alpha1 therapy for chronic hepatitis B patients. Intern Med. 2003;42:941-946. |
| 19. | Amarapurkar D, Das HS. Thymosin alpha in the treatment of chronic hepatitis B: an uncontrolled open-label trial. Indian J Gastroenterol. 2002;21:59-61. |
| 20. | Andreone P, Fiorino S, Cursaro C, Gramenzi A, Margotti M, Di Giammarino L, Biselli M, Miniero R, Gasbarrini G, Bernardi M. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antiviral Res. 2001;49:75-81. |
| 21. | von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599-605. |
| 22. | von Herbay A, Stahl W, Niederau C, von Laar J, Strohmeyer G, Sies H. Diminished plasma levels of vitamin E in patients with severe viral hepatitis. Free Radic Res. 1996;25:461-466. |
| 23. | Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380-1386. |
| 24. | Bortolotti F, Cadrobbi P, Crivellaro C, Guido M, Rugge M, Noventa F, Calzia R, Realdi G. Long-term outcome of chronic type B hepatitis in patients who acquire hepatitis B virus infection in childhood. Gastroenterology. 1990;99:805-810. |
| 25. | Gregorio GV, Jara P, Hierro L, Diaz C, de la Vega A, Vegnente A, Iorio R, Bortolotti F, Crivellaro C, Zancan L. Lymphoblastoid interferon alfa with or without steroid pretreatment in children with chronic hepatitis B: a multicenter controlled trial. Hepatology. 1996;23:700-707. |
| 26. | Chang MH, Sung JL, Lee CY, Chen CJ, Chen JS, Hsu HY, Lee PI, Chen DS. Factors affecting clearance of hepatitis B e antigen in hepatitis B surface antigen carrier children. J Pediatr. 1989;115:385-390. |
| 27. | Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990;52:557-563. |
| 28. | Wang Y, Huang DS, Liang B, Watson RR. Nutritional status and immune responses in mice with murine AIDS are normalized by vitamin E supplementation. J Nutr. 1994;124:2024-2032. |
| 29. | Bertoletti A, D'Elios MM, Boni C, De Carli M, Zignego AL, Durazzo M, Missale G, Penna A, Fiaccadori F, Del Prete G. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997;112:193-199. |
| 30. | Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. |
| 31. | Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857-861. |
| 32. | EASL International Consensus Conference on Hepatitis B. 13-14 September, 2002: Geneva, Switzerland. Consensus statement (short version). J Hepatol. 2003;38:533-540. |
| 33. | Mutimer D, Naoumov N, Honkoop P, Marinos G, Ahmed M, de Man R, McPhillips P, Johnson M, Williams R, Elias E. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998;28:923-929. |
| 34. | Ballauff A, Schneider T, Gerner P, Habermehl P, Behrens R, Wirth S. Safety and efficacy of interferon retreatment in children with chronic hepatitis B. Eur J Pediatr. 1998;157:382-385. |
| 35. | Kappus H, Diplock AT. Tolerance and safety of vitamin E: a toxicological position report. Free Radic Biol Med. 1992;13:55-74. |