Published online Dec 21, 2008. doi: 10.3748/wjg.14.7183
Revised: August 17, 2008
Accepted: August 24, 2008
Published online: December 21, 2008
AIM: To investigate if contrast-enhanced ultrasono-graphy (CE-US) is useful for determining treatment efficacy and outcome in the early stages of pancreatic cancer chemotherapy by assessing changes in intratumor hemodynamics using CE-US with a contrast agent.
METHODS: The subjects were 34 patients with unresectable advanced pancreatic cancer treated by chemotherapy. CE-US was assessed after every treatment (course) completion under the same conditions, and patients were divided into two groups according to the intratumor enhancement pattern: Vascular rich (R) group and vascular poor (P) group.
RESULTS: After the second course of treatment, R group in intratumor hemodynamics had 18 patients, and P group had 16 patients. The reduction rates of serum CA19-9 level after chemotherapy which decreased to half or less of the baseline level were 2/15 (0.1%) in P group, but 11/16 (69%) in R group (P = 0.006). When the mean number of courses of chemotherapy and outcome were compared, P group had a mean number of courses of 4.9 (R group, 10.2) and mean survival time (MST) of 246 d (R group, 402 d), showing that outcome was significantly better in R group (P = 0.006).
CONCLUSION: CE-US revealed that the change in intratumor blood flow correlated with both serum CA19-9 level and outcome. Patients with serum CA19-9 that decreased to less than half the baseline level, and patients with an abundant intratumor blood flow, had a significantly better outcome. Thus, CE-US is potentially useful for evaluating treatment efficacy and outcome in the early stages of pancreatic cancer chemotherapy.
- Citation: Sofuni A, Itoi T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J Gastroenterol 2008; 14(47): 7183-7191
- URL: https://www.wjgnet.com/1007-9327/full/v14/i47/7183.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7183
| Hemodynamic change in CE-US | No. of cases (n = 34) | CEM (n = 18) | CEM + CDDP (n = 13) | Chemo + radiation (n = 3) | Response | CA19-9 reduction of less than half (n = 31) | Average dosage (course) | Outcome (d) | ||||
| CR | PR | SD | PD | |||||||||
| R group | Total | 18 | 9 | 8 | 1 | 1 | 5 | 10 | 2 | 11 (61%)a | 10.2a | 402.0 |
| Increase (HI) | 12 | 6 | 5 | 1 | 1 | 3 | 8 | 0 | 8 (67%)a | 10.7 (6-18)a | 411.6 (239-6.4)a | |
| No change (II) iso-iso | 3 | 3 | 0 | 0 | 2 | 2 | 2 | 3 (50%)a | 9.0 (1-27)a | 383.5 (109-831)a | ||
| P group | Total | 16 | 9 | 5 | 2 | 0 | 1 | 6 | 9 | 2 (0.1%)a | 4.9a | 246.0a |
| No change (HH) hypo-hypo | 16 | 9 | 5 | 2 | 0 | 1 | 6 | 9 | 2 (0.1%) | 4.9 (1-12) | 246.0 (119-351) | |
| Decrease (IH) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The yearly mortality due to pancreatic cancer exceeds 200 000[1] worldwide and is increasing. Even with recent advances in diagnostic image technology, most cases of pancreatic cancer are only discovered at an unresectable stage, when the prognosis is poor and the 5-year survival rate is no more than 1%[1]. To date, multimodality therapy consisting of chemotherapy with 5-fluorouracil (5-FU)[2-7] and radiotherapy[8-10] has been the standard treatment for unresectable pancreatic cancer. Since the results of this treatment have not been satisfactory, gemcitabine (GEM) (Eli Lilly Japan, Kobe, Japan) has recently become the standard chemotherapy medication for unresectable pancreatic cancer, and GEM is expected to both increase antitumor efficacy[11-15] and improve clinical benefit response (CBR)[13].
The present study seeks to determine the antitumor effects of GEM in the early stages of treatment. To do so, we compared the enhancement patterns of pancreatic tumors visualized by contrast-enhanced ultrasonography (CE-US) using a microbubble contrast agent[16,17]. We investigated whether assessment of changes in intratumor hemodynamics by CE-US is useful for determining treatment efficacy and prognosis in the initial stages of chemotherapy for pancreatic cancer.
The subjects were 34 patients (17 Stage III and 17 Stage IV) with performance status 0-2 unresectable advanced pancreatic cancer that had visited this hospital and had undergone CE-US over the past 5 years. Staging of the disease was in accordance with The International Union Against Cancer (UICC) staging system (6th edition). The drugs and doses used were 1000 mg/m2 for GEM and 10 mg/m2 for cisplatin (CDDP). Seven patients showed adverse effects of GEM treatment, so the dose was reduced to 800 mg/m2. One course of treatment consisted of the following: GEM alone given to 18 patients, GEM + CDDP given to 13 patients, and GEM + CDDP with radiotherapy (50-60 Gy) given to three patients once per week for three weeks followed by a one-week rest. In addition, a histological diagnosis by endoscopic ultrasonography-fine needle aspiration (EUS-FNA), which has been reported to be useful[18], was performed for all the subjects prior to the chemotherapy treatment. After the chemotherapy treatment of patients who had received four courses or more, re-biopsy was again performed for eight patients that gave informed consent to do so. All patients provided written informed consent before the study. Our Institutional Review Board reviewed and approved the entire study.
The ultrasound equipment used was either a Sequoia 512 (Siemens-Acuson; Mountain View, CA, USA) or Aplio (Toshiba Medical Systems, Tokyo, Japan). Levovist R (2.5 g) (Shering; Tokyo, Japan) was prepared at a dose of 300 mg/mL, and a bolus of 7 mL was injected through the median cubital vein at a speed of 1 mL/s. After detailed observation of the lesion in B-mode, the vascular image was depicted for 60 s after injection of the contrast agent, at a speed of 5 frames/s (fps) with breath held, focusing on the blood flow dynamics in the lesion and surrounding pancreatic tissue. Thereafter, perfusion images were depicted for 180 s after injection with intermittent transmissions for periods of 2-10 s to observe the enhancement of the lesion. The color Doppler gain level was standardized as 50. We observed the blood vessels of the lesion in the vascular image, and the actual perfusion of the lesion through the capillaries in the perfusion image.
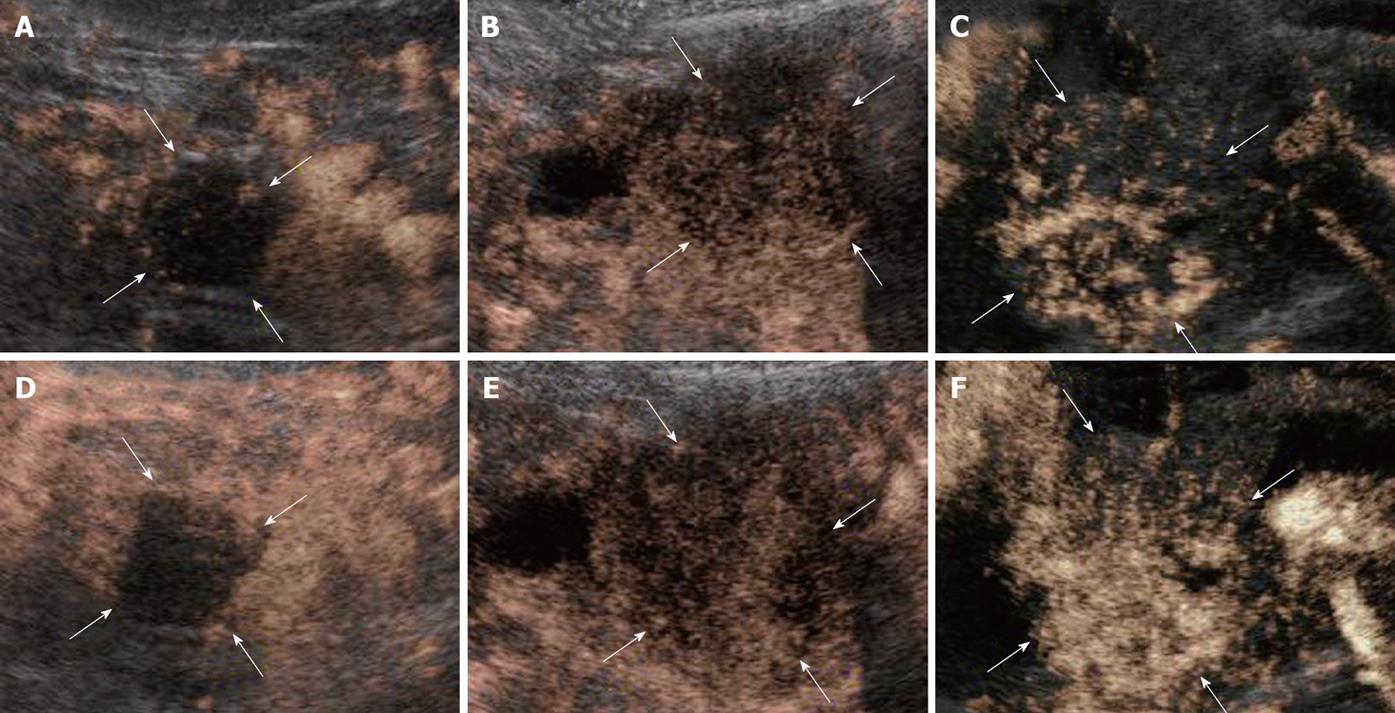
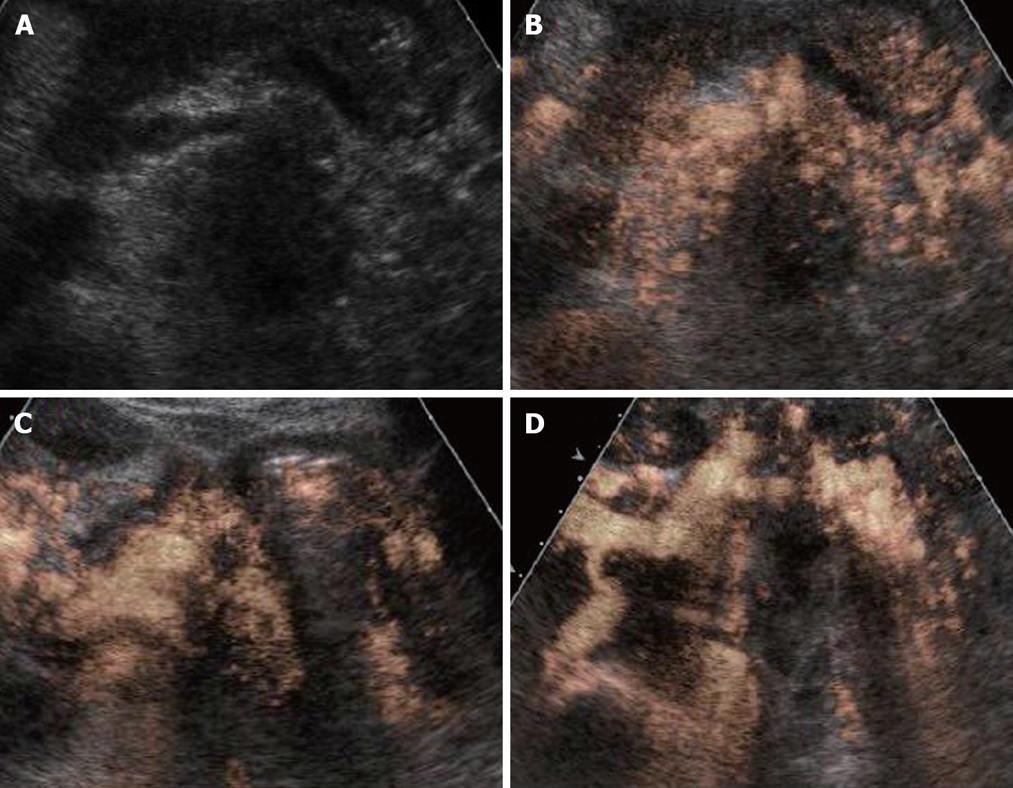
According to our previous report[16], both the vascular and perfusion images of the tumor blood flow signal were compared with the non-tumor regions of pancreas and classified depending on the signal intensities into the following three groups: hypervascularity (hyper), isovascularity (iso), and hypovascularity (hypo) (Figure 1).
CE-US enhancement patterns after chemotherapy were classified into the following patterns according to our previous assessment prior to the present study. CE-US was assessed after every treatment (course) completion under the same conditions, and patients were divided into four types: an increased intratumor blood flow type (from hypo to iso, HI type); an abundant intratumor blood flow that remained the same before and after treatment type (from iso to iso; II type), a no increase in intratumor blood flow type (from hypo to hypo, HH type), and a decreased intratumor blood flow type (from hyper/iso to hypo, IH type). Furthermore, these four types were further divided into two groups; a vascular rich (R) group of the HI and II types with a high post-treatment intratumor blood flow, and vascular poor (P) group of the HH and IH types with a poor post-treatment intratumor blood flow. The assessment of intratumor blood flow luminance was performed at the same time as the analysis of luminance where the midline of the tumor, which was the region of interest (ROI), was compared over time with adjacent non-tumor regions using Adobe Photoshop (Adobe Systems; San Jose, CA). This was done to measure the amount of blood flow objectively. When the luminance score (LS) (luminance of tumor area divided by luminance of normal area) is 20% or less, there is the possibility of increased luminance due to artifacts. Therefore, a conclusion of increased blood flow was reached if the LS increased by 20% or more compared to the baseline value. The images for all the patients were recorded and luminance evaluated from the same images by three physicians (T.I., T.K., and F.M.) experienced in CE-US.
Treatment efficacy and outcome were evaluated from the viewpoint of serum CA19-9 level and CE-US enhancement pattern in the R and P groups. The definition of response to treatment was evaluated after the second course of chemotherapy.
Student’s t test, Fisher’s exact test, Wilcoxon’s test, and the log-rank test were used for the statistical analysis. A P value < 0.05 was considered to indicate a statistically significant difference.
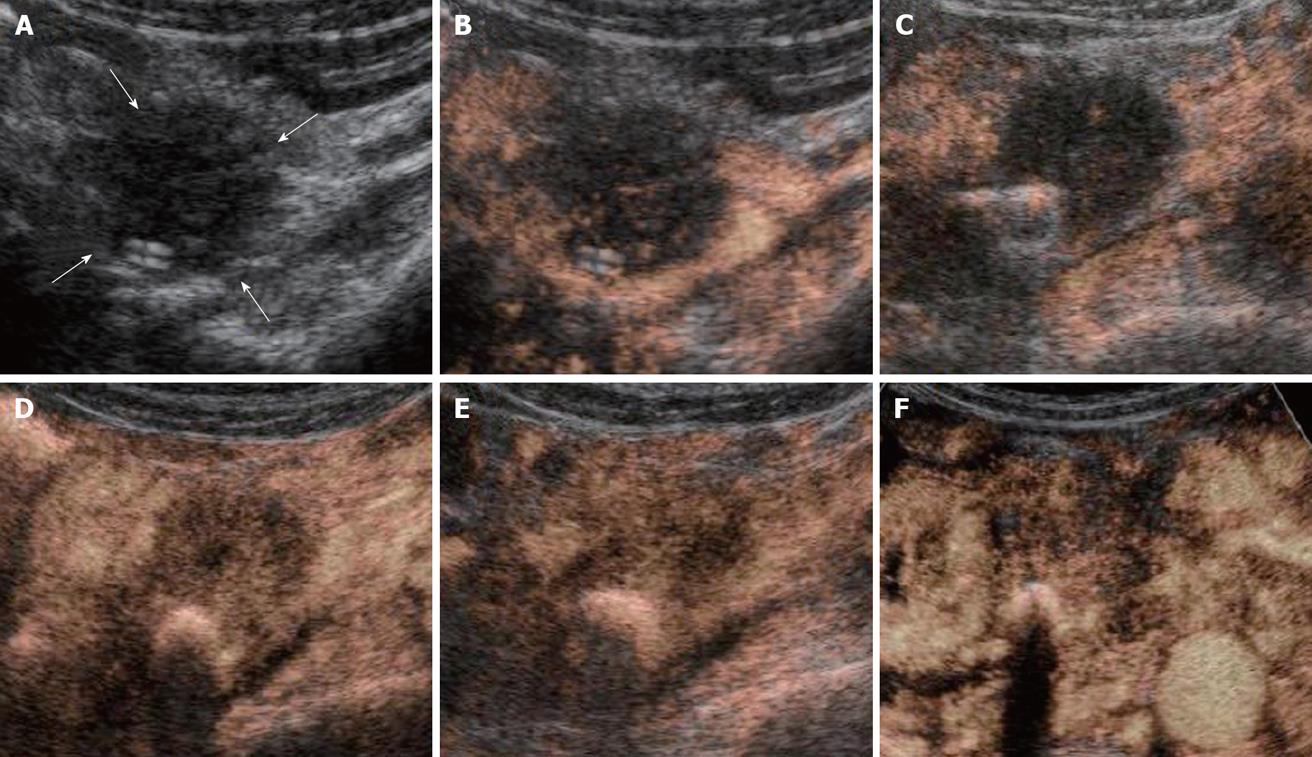
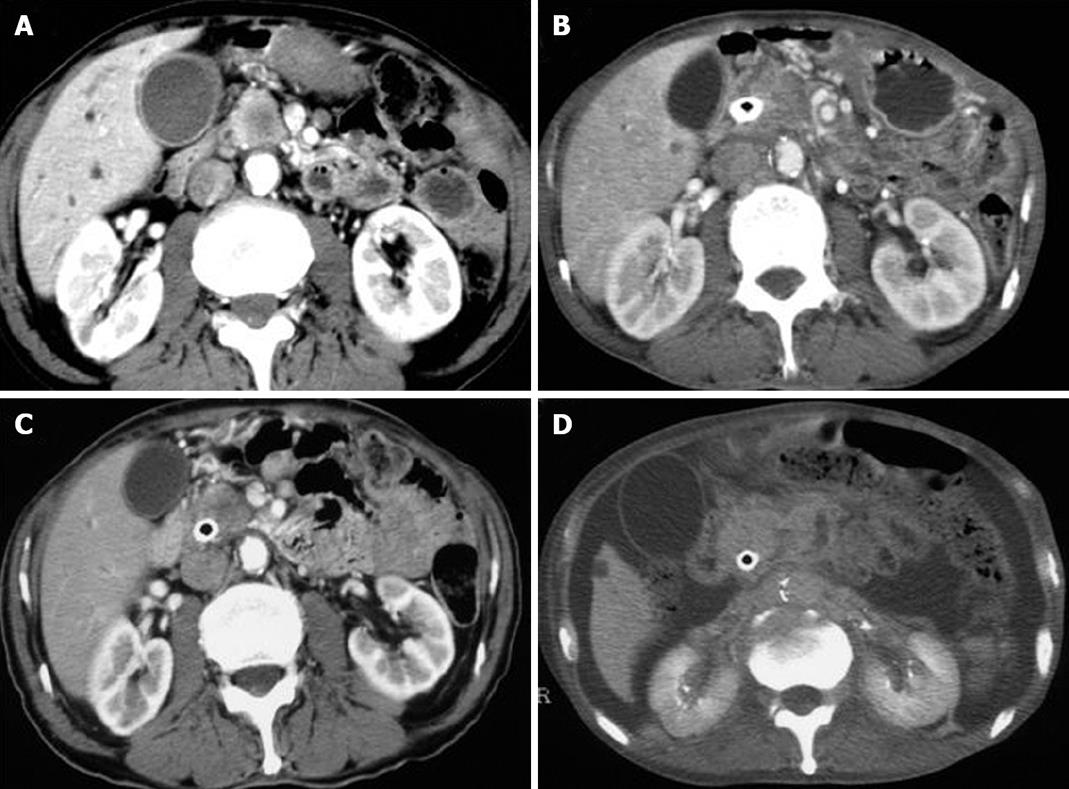
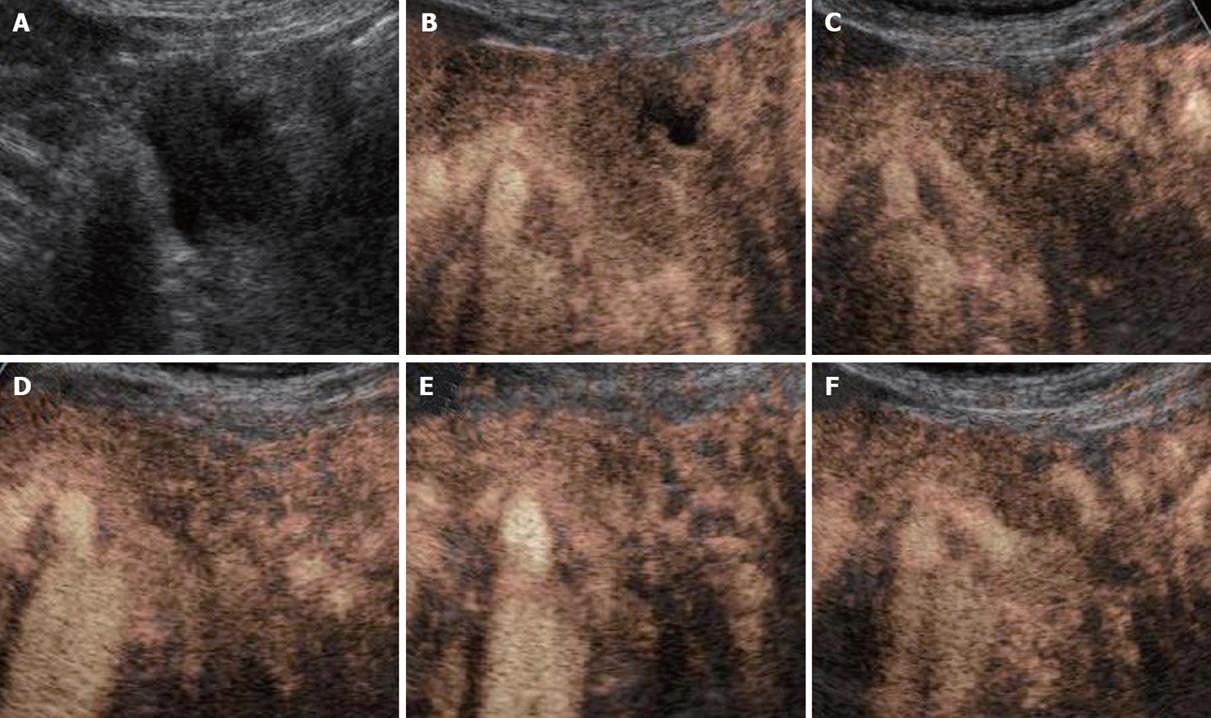
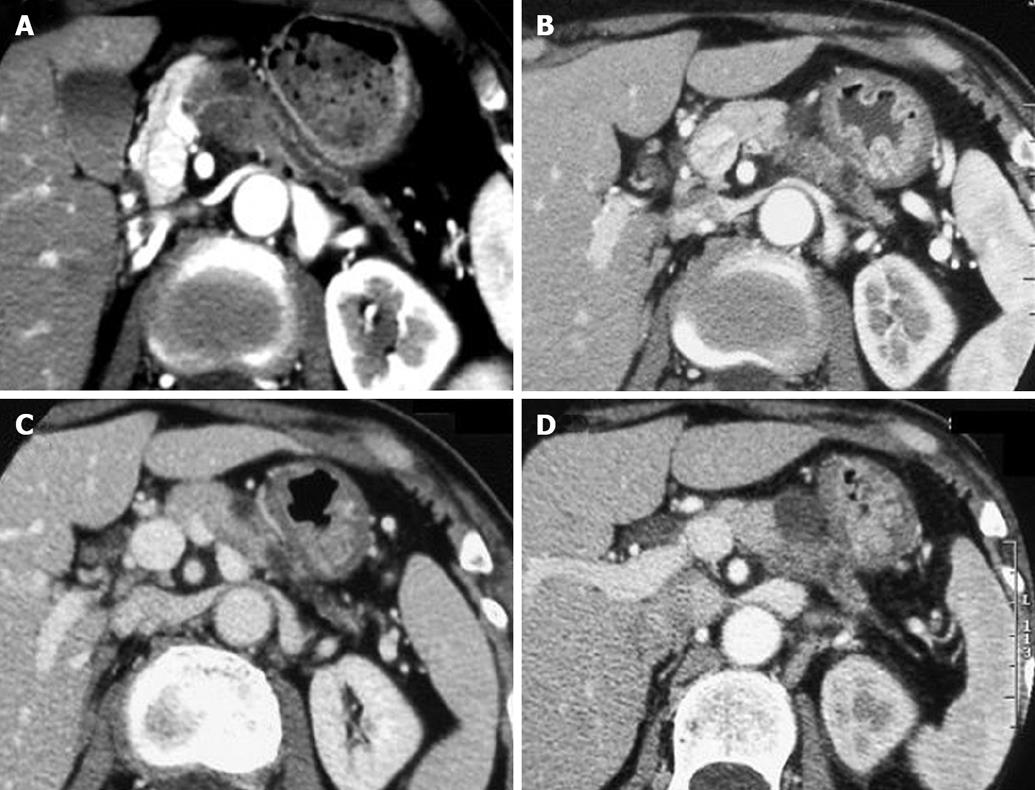
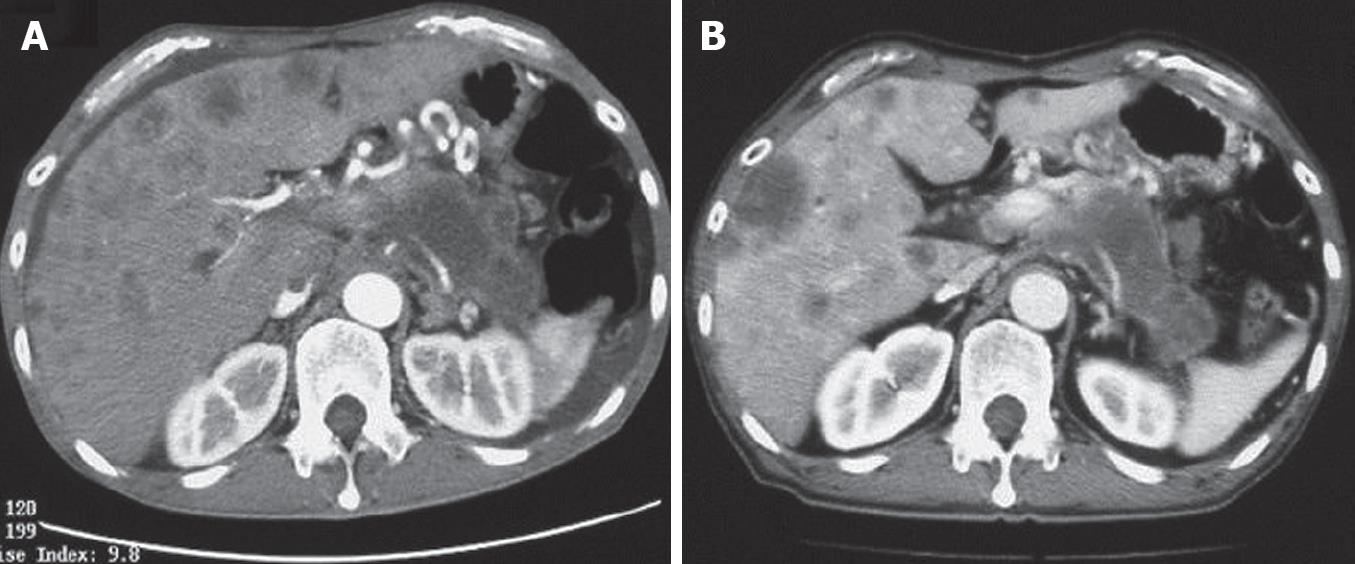
All the tumors were visualized in B-mode by CE-US, and mean tumor diameter was 39.4 mm (15-80 mm). When the CE-US enhancement patterns of the tumors before and after treatment were compared, intratumor blood flow before treatment was of the hypovascularity type in 28 (82%) patients and of the isovascularity type in 6 (18%) patients. After treatment, changes in intratumor hemodynamics after the second course were seen in 12 (35%) patients, all of whom had increased intratumor blood flow (HI type). There was no change in the hemodynamics in 22 (65%) patients (16 in the HH type and 6 in the II type). Blood flow did not decrease in any of the patients. Twelve patients in the HI type and 6 in the II type, making a total of 18 (53%) patients, were combined to form the R group. The CE-US and CT changes over time in the R group are shown in Figures 2, 3, 4, and 5. Similarly, for the P group (HH type), the CE-US and CT changes over time for 16 patients are shown in Figures 6 and 7. After treatment, there was increased imaging performance by both CE-US and CT in the HI type, in other words, the changes after treatment showed correlation in terms of images in 33% (4/12) patients. In addition, there was no significant difference in the tissue types.
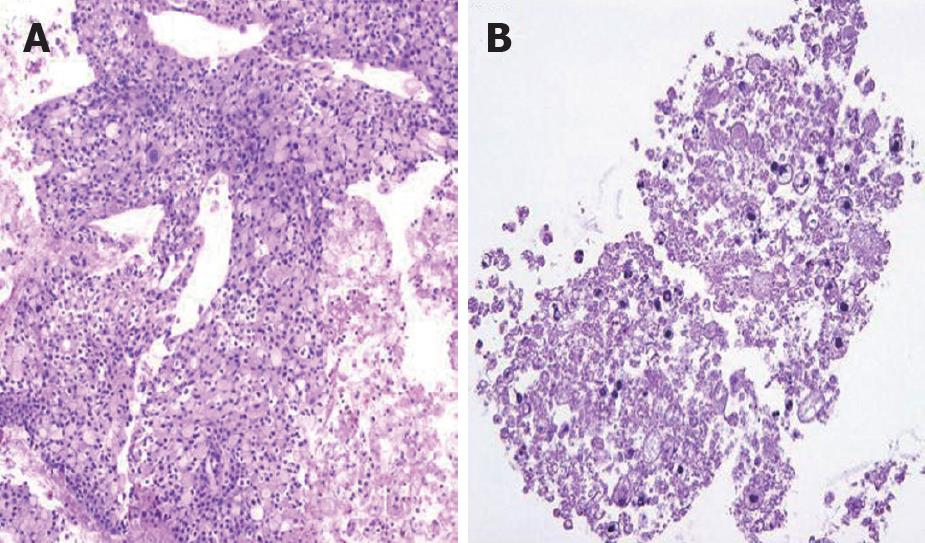
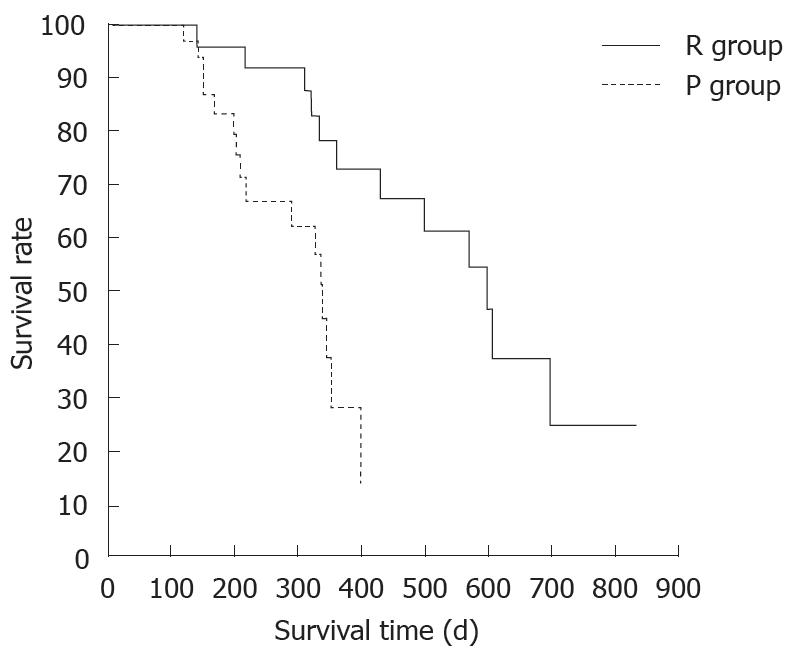
There was no difference between the R and P groups in relation to performance status or chemotherapy regimen. When mean tumor diameter before and after treatment in the R and P groups were compared, the values before treatment were 37.2 mm vs 41.8 mm and after treatment were 31.9 mm vs 37.5 mm, with no significant difference between the groups (P = 0.891). Tumor response based on Response Evaluation Criteria In Solid Tumors (RECIST) in the R group was rated as a complete response (CR) in one patient,partial response (PR) in 5 patients, stable disease (SD) in 10 patients and progressive disease (PD) in 2 patients. In the P group, results were PR in one patient, SD in 6 and PD in 9 patients. Although there was no significant difference between the groups, the response rate in the R group was high compared with P group (33% vs 6%, P = 0.051). These results are shown in Table 1. The grade of histological differentiation in the R group was well-moderately differentiated type in 12 patients,poorly differentiated medullary type in five patients and poorly differentiated scirrhous type in one patient. In the P group, it was well-moderately differentiated adenocarcinoma in 16 patients. There was no significant difference in grade of histological differentiation between the two groups (P = 0.072). In the 8 patients (6 in group R and 2 in group P) that had post-treatment tissue biopsy, the histological findings revealed only necrotic tissue except in one R group case showing viable malignant tissue (Figure 8). The patients whose serum CA19-9 level decreased to half the baseline level or less were 2/15 (0.1%) in group P but 11/16 (69%) in group R, showing a significant decrease in group R [P = 0.006; 95% confidence interval (CI), 41-88]. When mean courses of chemotherapy and outcome were compared, group P had a mean course number of 4.9 (range, 1-12) and mean survival time (MST) of 246 d (range, 119-351 d), whereas group R had a mean course number of 10.2 (range, 1-27) and MST of 402 d (range, 109-831 d), showing that outcome tended to be significantly better in group R (P = 0.006, 95% CI, 48-265). These results are shown in Table 1. The correlation between CE-US imaging pattern and MST based on these results is presented as a Kaplan Meier survival curve (Figure 9).
In this investigation, we demonstrated that patients with abundant intratumor blood flow (the R group) had significantly better GEM treatment results than the P group with a poor intratumor blood flow (P group vs R group; mean number of treatment courses: 4.9 vs 10.2; MST: 246 d vs 402 d). Patients with increased intratumor blood flow were commonly seen after the second course of treatment. With regard to tumor response, the GEM response rate in the R group was 33.3% while that in the P group was no more than 6.3%.
Halm[19] and Heinemann et al[20] reported good treatment efficacy in patients where serum CA19-9 level decreased by 20% or more of the baseline value. In the present study, serum CA19-9 level decreased to half the baseline value or less in 69% of patients in R group, where treatment efficacy was good. However, in P group, the decrease was only 0.1%. Serum CA19-9 is the sialylated Lewis blood group antigen that is expressed by the monoclonal antibody 1116 NS 19-9[21]. When this antigen is negative, an increase in serum CA19-9 level cannot be seen, making a clear interpretation difficult. These results suggest that changes in intratumor blood flow after treatment, as revealed by CE-US, are related to serum CA19-9 level and prognosis, and that prognosis is good in patients with serum CA19-9 levels that decrease to half the baseline level or less after treatment, and those with abundant intratumor blood flow.
GEM became commercially available in the late 1990s as a chemotherapy agent for unresectable pancreatic cancer, and is now positioned as a first-choice drug. We have used GEM as a primary chemotherapy agent but have sometimes experienced cases where the treatment has not been effective. Although the reason for that is not clear, recently there are reports that gemcitabine resistance has been associated with the ribonucleotide reductase subunit M2 (RRM2)[22]. If the status of treatment efficacy can be determined in the early stage of antitumor drug treatment, more effective chemotherapy can be selected. Physicians could then switch patients to other drugs, and select other treatment options such as concomitant therapy or combined treatment with radiotherapy. The burden that unnecessary anticancer drug treatments place on the body, as well as the time wasted, could thus be avoided.
Based on our previous investigations[16,17] of the CE-US imaging patterns of pancreatic tumor disease, it was assumed that the qualitative intratumor histological changes that were caused by treatment were reflected in the hemodynamics seen in CE-US. Therefore, CE-US could be applied to the evaluation of treatment efficacy after chemotherapy of pancreatic cancer.
The CE-US imaging patterns of pancreatic ductal cancer are basically classified into three types-hypo, iso, and hypervascular-with the most common pattern being hypovascularity[16]. Although the extent of the patterns in the tumors differed, a punctate or dendritical blood flow signal was seen in most patients. This indicates that the pancreatic tumor is an ischemic tumor, but demonstrates that much of the blood flow is at the capillary level. In the relationship between CE-US imaging pattern and histological differentiation, tissue type in the case of a hypovascularity pattern was moderately or poorly differentiated adenocarcinoma (scirrhous type). In the case of an isovascularity pattern, the tissue type was poorly differentiated adenocarcinoma (medullary type), whereas in the case of a hypervascularity pattern it was papillary adenocarcinoma[17]. These differences in imaging pattern are related to inflammatory changes, tumor development morphology and grade of histological differentiation, fibrosis and amount of interstitial connective tissue, vascular occlusion and degree of patency due to invasion of tumor vessels, and density.
Pancreatic cancers are generally anemic tumors and so respond poorly to anticancer treatment. It has become possible to visualize the microvascular flow in tumors by CE-US, and it is now evident that much of the intratumor blood flow is at the capillary level. With an abundant intratumor blood flow, more drugs will readily enter the tumor and thus increase treatment efficacy. The bubble diameter of the contrast agent is 2-3 μm, which is the same as the diameter of the capillaries that can be visualized. It is therefore preferable for the diameter of the particle system of anticancer drugs to be lower than that. According to the manufacturer (Eli Lilly Japan), the particle diameter of GEM is 0.2 μm or less. This is also important from the perspective of tissue transferability; GEM migrates up to the capillary level of tumors and so is assumed to show antitumor effect in tissues. The increase in intratumor blood flow in CE-US may be the reason for the intratumor histological changes and changes at the capillary level. The post-treatment results on EUS-FNA tissue showed that the tumor had changed into necrotic tissue and had decreased in volume. A reactive inflammatory change is triggered, causing disintegration of the tumor, which then becomes necrotic tissue. Furthermore, the volume of the tumor decreases and there is improvement in vascular occlusion and exclusion caused by tumor exclusion and vascular invasion. This is assumed to be the mechanism whereby intratumor blood flow improves. However, there was no significant difference between antitumor efficacy and grade of histological differentiation in this study, which suggests that antitumor efficacy not only correlates with the grade of histological differentiation, but also with other factors such as the amount of interstitial connective tissue and density.
In this study, the CT and CE-US imaging patterns before and after treatment were not always in concordance. The rate of concordance of the imaging patterns before treatment was 92% (31/34 patients), and after treatment it was 76% (26/34 patients). Moreover, in the R group, where treatment efficacy was good and CE-US revealed abundant blood flow, the CT imaging after treatment did not necessarily show change. In the HI type in particular, only 33% (4/12 patients) showed a correlation in imaging changes after treatment. The reasons for this include the fact that the contrast agents for CT and those for CE-US are different; the CT agents are predisposed to extravascular interstitial leakage, and there is a difference in the imaging time phase of the agents. There were some cases in which there was no change in tumor volume and enhancement pattern of CT after the treatment, but the outcome was comparatively good. The CE-US of such patients commonly revealed abundant intratumor blood flow, and also it showed that such patients were good outcome in our present study. Therefore, there was a difference of assessment between CT and CE-US.
At present, although the UICC guidelines suggest that antitumor effects should be evaluated based on volume change as estimated by CT, such evaluation is sometimes questionable. The results of the present investigation demonstrated that, apart from volume change by CT, there is the possibility that the CE-US imaging pattern is also one important parameter in the evaluation of the post-treatment effect. It can at least be said that CT-based estimation of antitumor effect leaves much to be desired. Moreover, there will be a problem of radiation exposure related to CT in the future. However, the role of CE-US imaging in prognostic evaluation needs to be investigated further in a larger patient cohort.
In conclusion, CE-US revealed that the change in intratumor blood flow after GEM treatment was correlated with serum CA19-9 level and outcome. Patients with serum CA19-9 levels decreasing to less than half the baseline level, and patients with an abundant intratumor blood flow, tended to have a good outcome. Thus, CE-US is potentially useful for evaluating treatment efficacy and outcome in the early stages of pancreatic cancer chemotherapy.
The yearly mortality due to pancreatic cancer exceeds 200 000 worldwide and is increasing. Even with recent advances in diagnostic image technology, most cases of pancreatic cancer are only discovered at an unresectable stage, when the prognosis is poor and the 5-year survival rate is no more than 1%.
To date, multimodality therapy consisting of chemotherapy with 5-fluorouracil (5-FU) and radiotherapy has been the standard treatment for unresectable pancreatic cancer. Since the results of this treatment have not been satisfactory, gemcitabine (GEM) has recently become the standard chemotherapy medication for unresectable pancreatic cancer, and GEM is expected to both increase antitumor efficacy and improve clinical benefit response (CBR).
The present study seeks to determine the antitumor effects of GEM in the early stages of treatment. We compared the enhancement patterns of pancreatic tumors visualized by contrast-enhanced ultrasonography (CE-US) using a microbubble contrast agent. We investigated whether CE-US is useful for determining treatment efficacy and outcome in the early stages of pancreatic cancer chemotherapy by assessing changes in intratumor hemodynamics using CE-US with a contrast agent.
CE-US revealed that the change in intratumor blood flow after GEM treatment was correlated with serum CA19-9 level and outcome. Patients with serum CA19-9 levels decreasing to less than half the baseline level, and patients with an abundant intratumor blood flow, tended to have a good outcome.
It is an interesting paper. The study revealed that CE-US is potentially useful for evaluating treatment efficacy and outcome in the early stages of pancreatic cancer chemotherapy.
Peer reviewer: Dr. Aydin Karabacakoglu, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Zhong XY L- Editor Logan S E- Editor Zheng XM
| 2. | Hansen R, Quebbeman E, Ritch P, Chitambar C, Anderson T. Continuous 5-fluorouracil (5FU) infusion in carcinoma of the pancreas: a phase II study. Am J Med Sci. 1988;295:91-93. |
| 3. | Kovach JS, Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ. Proceedings: A controlled study of combined 1,3-bis-(2-chloroethyl)-1-nitrosourea and 5-fluorouracil therapy for advanced gastric and pancreatic cancer. Cancer. 1974;33:563-567. |
| 4. | Wiggans RG, Woolley PV, Macdonald JS, Smythe T, Ueno W, Schein PS. Phase II trial of streptozotocin, mitomycin-C and 5-fluorouracil (SMF) in the treatment of advanced pancreatic cancer. Cancer. 1978;41:387-391. |
| 5. | Evans TR, Lofts FJ, Mansi JL, Glees JP, Dalgleish AG, Knight MJ. A phase II study of continuous-infusion 5-fluorouracil with cisplatin and epirubicin in inoperable pancreatic cancer. Br J Cancer. 1996;73:1260-1264. |
| 6. | Rothman H, Cantrell JE Jr, Lokich J, Difino S, Harvey J, Ahlgren J, Fryer J. Continuous infusion 5-fluorouracil plus weekly cisplatin for pancreatic carcinoma. A Mid-Atlantic Oncology Program study. Cancer. 1991;68:264-268. |
| 7. | Crown J, Casper ES, Botet J, Murray P, Kelsen DP. Lack of efficacy of high-dose leucovorin and fluorouracil in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 1991;9:1682-1986. |
| 8. | Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705-1710. |
| 9. | Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751-755. |
| 10. | Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373-378. |
| 11. | Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999;85:1261-1268. |
| 12. | Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101-105. |
| 13. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. |
| 14. | Casper ES, Green MR, Kelsen DP, Heelan RT, Brown TD, Flombaum CD, Trochanowski B, Tarassoff PG. Phase II trial of gemcitabine (2,2'-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs. 1994;12:29-34. |
| 15. | Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA 3rd, Green MR, Tarassoff PG, Brown TD, Casper ES. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347-353. |
| 16. | Sofuni A, Iijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K, Itokawa F, Itoi T. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518-525. |
| 17. | Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854-859. |
| 18. | Itoi T, Takei K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Nakamura K, Moriyasu F, Tsuchida A, Kasuya K. Immunohistochemical analysis of p53 and MIB-1 in tissue specimens obtained from endoscopic ultrasonography-guided fine needle aspiration biopsy for the diagnosis of solid pancreatic masses. Oncol Rep. 2005;13:229-234. |
| 19. | Halm U, Schumann T, Schiefke I, Witzigmann H, Mössner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013-1016. |
| 20. | Heinemann V, Schermuly MM, Stieber P, Schulz L, Jüngst D, Wilkowski R, Schalhorn A. CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anticancer Res. 1999;19:2433-2435. |
| 21. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. |
| 22. | Itoi T, Sofuni A, Fukushima N, Itokawa F, Tsuchiya T, Kurihara T, Moriyasu F, Tsuchida A, Kasuya K. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42:389-394. |