INTRODUCTION
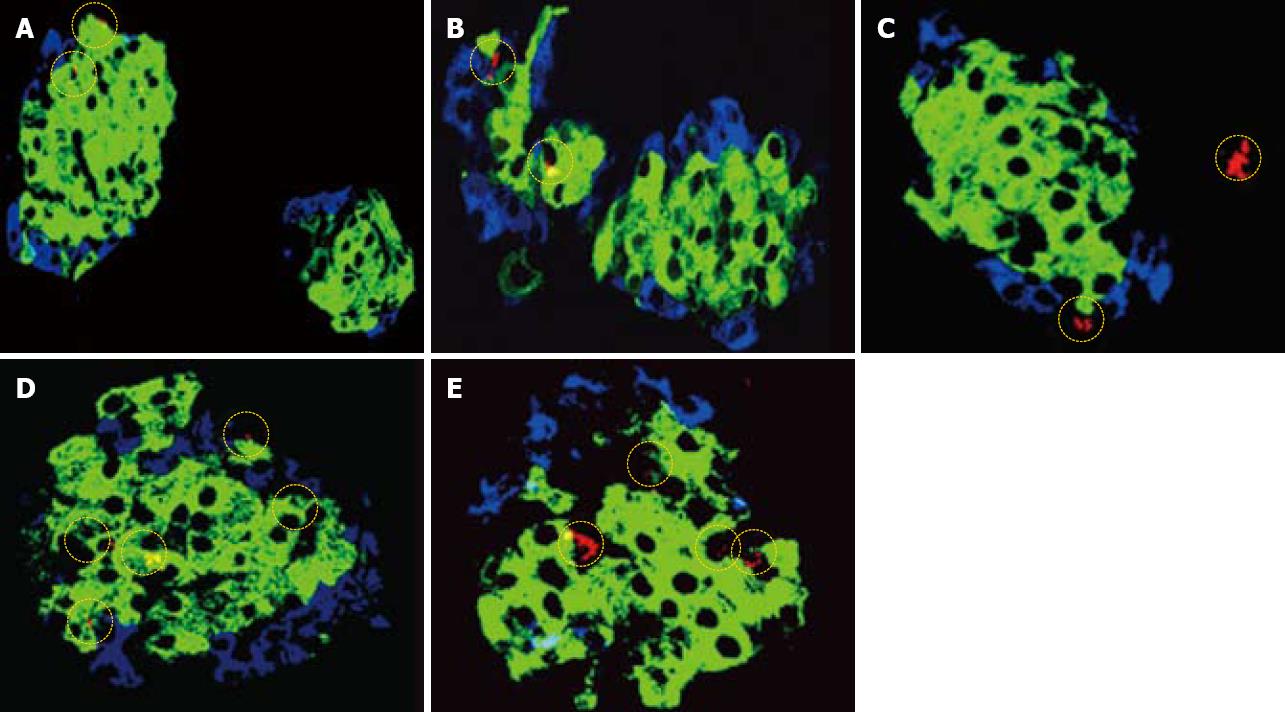
Figure 1 Immunolocalization of nestin in mice pancreases at different ages.
A: Immunohistochemical localization of Nestin (red), Insulin (green), glucagon (blue) in three-day-old mouse pancreatic section, the presence of nestin is more towards the periphery of the endocrine fraction, magnification (× 400); B: One-week-old mouse pancreatic section, the presence of nestin is more towards the periphery of the endocrine fraction, magnification (× 400); C: Two-week-old mouse pancreatic section showing Nestin staining in both the exocrine as well as endocrine fraction, magnification (× 400); D: Four-week-old mouse pancreatic section showing Nestin staining only in the endocrine fraction confining more towards the periphery of the insulin stained cells, magnification (× 400); E: Eight-week-old mouse pancreatic section showing the Nestin localization predominant in the central region of the islets clusters where immunostaining for insulin was also significant, magnification (× 400).
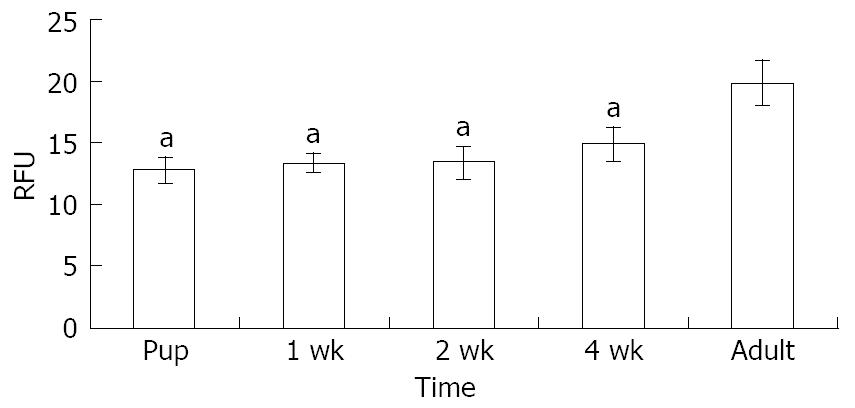
Figure 2 Quantitative relative fluorescence units of Nestin for different age groups performed in three different sections showing a higher level in the 8-wk-old pancreatic tissue.
aP < 0.05 in the adult as as compared to other four ages.
Diabetes results from an inadequate mass of functional pancreatic β-cells and such inadequacy can result from a lack of selective autoimmune destruction of pancreatic β-cells (type 1)[1,2] or a lack of compensation to overcome Insulin resistance (type 2)[3,4]. In addition, an intrinsic β-cell defect, fragility, or shortened life span may be other possible impairments underlying the pathophysiology of diabetes[5]. Recently, adult pancreatic tissue (exocrine/endocrine) has emerged as a promising source of pancreatic stem cells, which are capable of differentiating into functional insulin secreting cells (ISC) given an appropriate microenvironment[6,7]. The introduction of new methods for in vitro generation of β-cells from pancreatic tissue will help to provide greater numbers of viable ISC.
The promise of using adult tissue stem cells are attributed to their wide distribution in almost all tissues, modulation of their plasticity, ability to be modified genetically or reprogrammed and they are safer for transplantation[8]. They can also be immortalized or multiplied in culture for a number of passages and the ethical constraints on their use are minimal as compared to the use of embryonic stem cells[9]. Nestin has been unequivocally identified as a marker of neural stem cells or progenitors[10,11]. It is abundantly expressed in neuroepithelial cells during embryogenesis but it is nearly absent from all mature central nervous system cells[12,13]. In addition, nestin has been demonstrated in rat bone marrow[14], human embryonic stem cells[15,16], human islet explants[17], human fetal pancreas[18], and adult rat pancreas[19]. The varied expression and characterization of nestin positive cells (NPC) in different cell types is thought to have considerable functional significance during the developmental process. Despite several studies documenting the potential of NPC as stem cells/progenitors to differentiate into ISC, some studies have reported that NPC may not function as progenitors for ISC[20,21]. Nestin is an intermediate filament protein and might play a key role in imparting cytoskeletal functions to the cell[22]. In support of these reports, ductal epithelial cells (DEC), which comprise 10% of the pancreatic cells expressing the intermediate filament proteins cytokeratin 7 and 19, have been demonstrated to function as pancreatic progenitors due to their ability to generate ISC[7]. NPC present in the pancreas, in addition to imparting the structural functions, also participate in developmental regulation as progenitors, similarly to DEC[23].
Therefore, exploring in vitro system(s) for the precise characterization and propagation of NPC would be a feasible approach to harness the potential of NPC as progenitors capable of differentiation into ISC. The present investigation has been undertaken to characterize NPC in the pancreas of mice at different ages (3 d to 8 wk), which has not been previously reported. Hence, understanding the localization of NPC at different ages would help in identifying the source of the progenitors and can further facilitate their differentiation into ISC under an appropriate microenvironment.
MATERIALS AND METHODS
Experimental design
All animal experimental procedures were approved by the Institutional ethical committee on animal research. Male Swiss albino mice aged 3 d, 1, 2, 4 and 8 wk were obtained from the National Center for Laboratory Animal Sciences (NCLAS), National Institute of Nutrition, Hyderabad, India.
Tissue preparation
The animals were ethically sacrificed using CO2 asphyxiation and their pancreas were removed and washed with PBS. After removal of the adhered fat, the tissue was fixed overnight in Bouins solution at room temperature[24,25]. The fixed tissue was washed twice in 70% Ethanol and paraffin sections of 6-8 μm were cut using a microtome before being mounted on to poly-L-lysine coated slides. They were dried overnight at 37°C before proceeding to immunolocalization.
Sample preparation and immunolocalization for nestin/insulin/glucagon
The pancreatic sections from all the age groups were processed under identical conditions. The samples were de-paraffinized by heating at 60°C for 30 min. They were then dehydrated by passing through a series of decreasing concentration of ethanol (100%, 90%, 70%, 50%, and 30%). This was followed by washing for 2 min each with double distilled water and PBS. They were then blocked with 4% Horse serum at room temperature for 1 h. Subsequently they were incubated with primary antibody overnight at 4°C (Mouse anti Rat Nestin 1:200 BD Biosciences, USA, anti mouse glucagon 1:200 Santa Cruz and anti mouse insulin 1:500 Sigma, USA). After repeated washing with PBS containing Ca2+ and Mg2+, the slides were treated with a secondary antibody tagged with an appropriate fluorescent dye (Goat anti guinea pig alexa 488, Goat anti rabbit alexa 546, Goat anti mouse alexa 633, Molecular Probes, USA). The fluorescence images were captured using a confocal microscope (Leica SP5 series) and fluorescent intensities units (FIU) were corrected using appropriate controls (primary antibody controls). The FIU have been quantitated as relative fluorescent units (RFU) and the experiments have been carried out independently in three sets of mice.
Statistical test
Results are expressed as mean ± SE using three independent experiments. One-way analysis of variance (ANOVA) was used, followed by a post-hoc LSD test with SPSS software to determine the significance.
RESULTS
Immunolocalization of nestin in mice pancreases at different ages is shown in Figure 1. In the early phase of postnatal period (3-6 d pups), the NPC were localized more towards the periphery of the endocrine pancreas, as evident from the colocalization of insulin and glucagon with NPC and by the absence of NPC in the acinar fraction (Figure 1A and B). In the 2-wk-old mice, the NPC were seen both in the exocrine as well as the endocrine pancreas (Figure 1C). Interestingly, in the 4-wk-old mice, the NPC were confined to the endocrine pancreas and were located more towards the periphery along with the glucagon positive cells (Figure 1D). In the 8-wk-old pancreatic tissue, the NPC localized predominantly in the central region of the islets clusters, where immunostaining for insulin was also predominant (Figure 1E). The RFU for the localization of nestin is shown in (Figure 2) where the NPC showed predominance in the insulin enriched fraction by eight week.
DISCUSSION
The present observations unequivocally suggest heterogeneity in the distribution of NPC within pancreatic tissue at different ages of mice (3 d to 8 wk) and this could be of functional and developmental significance. During embryogenesis, an increased expression of nestin has been reported in the neuroepithelial stem cells[26]. In line with these studies, the role of nestin has been demonstrated in the process of cellular rearrangements in the undifferentiated cells[27,28]. Studies in human islet explants, rat pancreatic tissue and embryonic stem cells have explored nestin as a marker of pancreatic progenitor stem cells, which have the renewal property and can be regulated to differentiate into insulin and glucagon positive cells[29,30].
The immunohistochemical localization of NPC (predominantly in the insulin-enriched fraction) has been depicted as RFU in Figure 2. These observations show that the endocrine portion of the pancreas serves as the primary enriched source of progenitors such as NPC, which, like DEC, can be expanded and differentiated into ISC with the appropriate growth factors in their microenvironment. In the present study, the application of reliable and specific immunocytochemical technique using specific antibodies and confocal microscopy has enabled the identification of NPC associated/colocalized with insulin and glucagon cells.
We report for the first time the significant localization of NPC (progenitors) in the endocrine pancreas at different ages of the mice. With increasing age of mice, the colocalization of NPC is more predominant in the insulin positive cells in the central region, unlike that seen at other ages. Identification of the sources and understanding the conditions and factors within the microenvironment of the pancreatic stem cells will be of therapeutic importance for the generation of ISC, which may be useful in the management of diabetes.
COMMENTS
Background
The present study has been undertaken to investigate the precise localization and characterization of nestin positive cells (NPC) as a source of pancreatic progenitors that are capable of differentiating into insulin secreting cells (ISC). Nestin is an intermediate filament protein playing key role in imparting cytoskeletal functions to the cell and has been demonstrated as a progenitor in the neuronal tissue, bone marrow, embryonic stem cells, islet explants, fetal and adult pancreatic tissue.
Innovations and breakthroughs
This paper demonstrates the immunolocalization of NPC along with insulin and glucagon at different ages of mice for the first time.
Applications
Their data demonstrates the predominance of NPC in the insulin enriched fraction of 8-wk-old mice and these observations are significant due to the fact that NPC are one of the progenitors of adult pancreatic tissue. The NPC can be expanded and differentiated into ISC with the appropriate growth factors in its microenvironment.
Terminology
NPC standed for a pancreatic progenitor; ISC means the beta cells of islet that secrete insulin.
Peer review
The study investigates immunolocalization of nestin in the mouse pancreas. Nestin is a known marker of neural stem cells, which is also transiently expressed by many types of cells during development. Upon differentiation, nestin becomes downregulated. One best known instance of nestin expression in adult organisms, are the neuronal precursor cells of the subventricular zone. The study by Dorisetty et al suggests that nestin is localized to the endocrine pancreas. Based on this, the authors hypothesize that pancreatic endocrine tissue is a potent source of progenitor cells, which could be reprogrammed into insulin-producing cells and could be used for the treatment of diabetes. The idea of the study is interesting, and is supported by the literature data.