Published online Dec 7, 2008. doi: 10.3748/wjg.14.6954
Revised: November 12, 2008
Accepted: November 19, 2008
Published online: December 7, 2008
AIM: To infect mice with atypical Campylobacter concisus (C. concisus) for the first time.
METHODS: Three separate experiments were conducted in order to screen the ability of five clinical C. concisus isolates of intestinal origin and the ATCC 33237 type strain of oral origin to colonize and produce infection in immunocompetent BALB/cA mice. The majority of the BALB/cA mice were treated with cyclophosphamide prior to C. concisus inoculation to suppress immune functions. Inoculation of C. concisus was performed by the gastric route.
RESULTS: C. concisus was isolated from the liver, ileum and jejunum of cyclophosphamide-treated mice in the first experiment. No C. concisus strains were isolated in the two subsequent experiments. Mice infected with C. concisus showed a significant loss of body weight from day two through to day five of infection but this decreased at the end of the first week. Histopathological examination did not consistently find signs of inflammation in the gut, but occasionally microabscesses were found in the liver of infected animals.
CONCLUSION: Transient colonization with C. concisus was observed in mice with loss of body weight. Future studies should concentrate on the first few days after inoculation and in other strains of mice.
-
Citation: Aabenhus R, Stenram U, Andersen LP, Permin H, Ljungh Å. First attempt to produce experimental
Campylobacter concisus infection in mice. World J Gastroenterol 2008; 14(45): 6954-6959 - URL: https://www.wjgnet.com/1007-9327/full/v14/i45/6954.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6954
| Strain | Origin | Diagnosis | No. of experiment | Protein group | AFLP group |
| ATCC 33237 | Oral | Periodontitis | 1 and 3 | 1 | 1 |
| RH10776.98 | Intestinal | Healthy traveller | 1 and 3 | 2 | 2 |
| RH4204.98 | Intestinal | IBD | 2 and 3 | 2 | 2 |
| RH4482.98 | Intestinal | NHL and IBD | 2 | 2 | 1 |
| RH5097.98 | Intestinal | ALL (IR) | 2 | 2 | 2 |
| RH15690.98 | Intestinal | ALL (IR) | 2 | 2 | - |
| Day | Control group | Clinical strains | Type strain | |
| RH10776.981 | RH4204.98 | ATCC 33237 | ||
| 0 | 16.0 | 17.2 (0.60) | 16.9 (0.54) | 16.6 (0.50) |
| 1 | 16.4 | 17.5 (0.57) | 16.9 (0.30) | 16.6 (0.42) |
| 2 | 16.7 | 17.2 (0.20) | 16.4 (0.03) | 16.6 (0.27) |
| 3 | 16.8 | 17.0 (0.13) | 16.6 (0.06) | 15.4 (0.08) |
| 4 | 17.3 | 17.5 (0.17) | 16.7 (0.09) | 16.5 (0.20) |
| 5 | 17.5 | 17.3 (0.04) | 16.8 (0.11) | 16.6 (0.15) |
| 6 | 18.1 | 18.1 (0.12) | 17.6 (0.20) | 17.4 (0.21) |
| Stomach | Liver | Ileum | Jejunum | Colon | Stools | |
| Culture | 0 | 3 | 3 | 1 | 0 | 0 |
| PCR | 0 | 1 | 1 | 1 | 0 | 0 |
The importance of Campylobacter jejuni (C. jejuni) and C. coli in causing diarrhoeal disease worldwide is well established[1]. However, a possible enteric pathogenic role for Campylobacter spp. other than C. jejuni/coli has not been established as yet, however, the potential pathogen C. concisus is at present the subject of investigation concerning its role in human gastrointestinal disease[2]. Campylobacter concisus (C. concisus) has been found in high proportions in patients with diarrhoeal disease, especially in tertiary hospital settings[3-6]. Other studies have shown C. concisus to possess virulence factors including a haemolytic phospholipase and cytotoxic activity caused by the Cytolethal Distending Toxin (CDT) as described in C. jejuni[7,8].
The presence of C. concisus in the gastrointestinal tract has been described by some investigators as that of a commensal as no statistical differences were found between symptomatic and asymptomatic carriers[9]. However, the fact that C. concisus is a heterogeneous species with several subtypes and at least two genomo-species could in part account for the conflicting results[10-12]. To our knowledge, no reports of C. concisus in animal models have been presented.
Attempts to establish an animal model mimicking human campylobacteriosis using various animals have suffered from difficulties due to handling, lack of reproducibility, high costs and inadequate clinical pathology[13-17]. A major shortcoming of murine models is the inability to consistently reproduce the most common symptoms of enteritis following gastric inoculation, although sporadic colonization of the gastrointestinal tract has been achieved[18-20]. Colonization and development of gastrointestinal symptoms is augmented in immunodeficient or immune dysregulated mice and following pre-treatment with oral antibiotics or iron[21-24]. Consequently, no single animal model is widely accepted and applied in the study of Campylobacter infection. The objective of this study was to determine if five clinical C. concisus strains of intestinal origin and the type ATCC 33237 strain of oral origin could infect immunocompetent and immunodeficient mice and mimic human Campylobacter infections.
The C. concisus reference and type ATCC 33237 strain of oral origin and five clinical isolates of C. concisus (RH10776.98, RH4204.98, RH4482.98, RH5097.98, RH15690.98) all from humans with diarrhoeal disease, were used. Strain details are given in Table 1. The isolates for inoculation were recovered from frozen storage, plated on 5% blood agar plates containing yeast extract (1%) and incubated in a microaerobic atmosphere (50 mL/L O2, 100 mL/L H2, 850 mL/L N2) at 37°C for 48 h as described previously[25]. The bacteria were harvested directly from plates into sterile phosphate buffered saline (PBS), washed and resuspended in PBS to the required cell density (109 CFU/mL) determined by optical density.
Immunocompetent BALB/cA mice of both sexes (from the breeding colony of the University of Lund, Sweden), 6-7 wk old and with a mean weight of 16.7 g were used in the study. The mice were not defined as flora or specific pathogen free. The mice were housed in groups of a maximum of ten mice of equal gender per cage. All mice received vancomycin by intragastric intubation (0.3 mL, 80 μg/mL) three days prior to the C. concisus challenge. The mice had access, ad libitum, to water and standard food. Three days before the start of the experiments, faecal pellets were analysed for the presence of Campylobacter spp., and prior to all experiments 2-4 mice were sacrificed for baseline values with complete sampling including culture, PCR and histopathology of the stomach, liver, ileum, jejunum and colon tissue. All experiments were performed according to the recommendations of the Swedish Board of Animal Research and were approved by the Committee of Animal Ethics of the University of Lund.
The study consisted of three experiments involving a total of 132 BALB/cA mice. Experiment 1 involved 22 animals, divided into 10 groups and the animals were inoculated with the type ATCC 33237 strain and the clinical isolate 10776.98. Half the mice received an intraperitoneal injection of cyclophosphamide (100 μL/10 g) three days before inoculation with the C. concisus strains in order to suppress normal immune systems by disrupting the T-cell population. The cyclophosphamide treated and untreated mice were kept in separate cages. Two mice were used for baseline values. Experiment 2 involved 54 animals, divided into 10 groups and the animals were inoculated with four clinical strains (RH4204.98; RH15690.98; RH4482.98; RH5097.98) or sham dosed with PBS. Half the mice received an intraperitoneal injection of cyclophosphamide (100 μL/10 g) three days before inoculation with the C. concisus strains. Four mice were used for baseline values. Experiment 3 involved 56 animals, divided into 13 groups and the animals were inoculated with the type ATCC 33237 strain, two clinical strains (RH4204.98; RH10776.98) or sham dosed with PBS. All mice received an intraperitoneal injection of cyclophosphamide (100 μL/10 g) three days before inoculation with C. concisus strains. Four mice were used for baseline values.
Inoculation of mice was performed by direct intragastric administration of 0.3 mL 109 CFU/mL or 0.3 mL of PBS by gastro-oesophageal tube (outer diameter 0.1 cm). Mice were challenged with a total of three equal doses of C. concisus on three consecutive days.
All animals were examined daily for signs of distress. The consistency of faecal pellets was also noted. Body weights of all mice in the third experiment were measured daily. Mice were euthanized by inhalation of carbon dioxide on day 7 (study 1: 3 mice; study 2: 5 mice; study 3: 5 mice), day 13 (study 1: 2 mice; study 2: 3 mice; study 3: 5 mice) and day 35 (study 1: 2 mice; study 2: 2 mice; study 3: 3 mice), and sampling from the stomach, liver, jejunum, ileum and colon was performed.
Microbiological culture: Faecal and tissue samples were homogenized in PBS, plated onto blood agar containing yeast extract using the filter technique and incubated in a microaerobic environment for 48 h as previously described[25]. Identification was performed on phenotypic data according to On[26].
Detection by PCR: Specimens were stored at -18°C until examination. DNA was extracted from faecal and tissue samples using DNeasy tissue minikit (Qiagen, Ballerup, Denmark) using a clean room procedure. Organ tissues were vortexed and manually degraded to homogenize the solutions before DNA extraction. Primers and amplification cycles were prepared according to Bastyns et al[27]. The mixture consisting of one forward primer MUC1 (5'-ATGAGTAGCGATAAATTGGG-3'), and two reverse primers CON1 (5'-CAGTATCGGCAATTCGCT-3') and CON2 (5'-GACAGTATCAAGGATTTACG-3'). All C. concisus strains tested were PCR detectable when tested directly from agar plates prior to inoculation.
Specimens for histological examination were prepared throughout the study. Stomach, liver, small and large intestine were removed immediately after death, fixed in neutral buffered 4% formaldehyde, paraffin embedded and processed for histopathological evaluation. Sections were stained with haematoxylin and eosin and examined under a light microscope by an experienced pathologist. All examinations were performed blindly.
The loss of body weight in the various infected groups compared to the control group was evaluated using the Mann-Whitney U-test, with normalized weight ratios from day 1 of the experiment. Thus, for example, in Table 2 on day 2, the control group 0.7 (weight increase) is compared to ATCC 33237 0.0 (unchanged weight). Results were considered statistically significant when P < 0.05.
Culture: On day 7 of experiment 1, C. concisus was isolated from all three mice sacrificed in the cyclophosphamide-treated group, inoculated with the clinical isolate RH10776.98. All mice had positive liver and ileum cultures, whereas only one mouse had a positive jejunum culture (Table 3). Faecal pellets examined throughout the study were consistently negative. Tissue samples obtained on day 21 and day 56 were negative for C. concisus. Isolation of C. concisus was not obtained during the two subsequent experiments (2 and 3).
PCR: PCR testing of the samples yielded comparable results on day 7 of experiment 1, as all three mice sacrificed in the cyclophosphamide-treated group, inoculated with the clinical isolate RH10776.98, were PCR positive when analysing ileum tissue (Tables 2 and 3). Using PCR, C. concisus was not detected from liver or jejunum tissue. Tissue samples obtained on day 21 and day 56 were not positive for C. concisus. PCR results were consistently negative in the two subsequent experiments (2 and 3).
Stomach: No evidence of inflammation or infection was noted throughout the study.
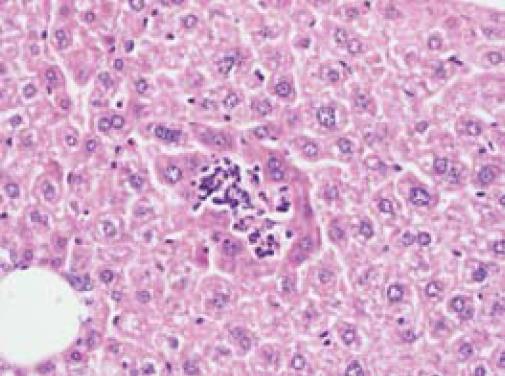
Liver: One mouse inoculated with the clinical isolate RH10776.98 and one mouse inoculated with the type ATCC 33237 strain, both from non-treated groups in experiment 1, harboured microabscesses in their livers (Figure 1). Megakaryocytes were occasionally noted in the livers of cyclophosphamide-treated groups, including the controls, but not in untreated controls. The three C. concisus-positive livers all showed evidence of megakaryocytes and haematopoiesis.
Jejunum: No evidence of inflammation or infection was found, although oedema of intestinal villi was detected in the cyclophosphamide-treated control group. One mouse inoculated with the clinical C. concisus isolate RH15690.98, showed infiltration of lymphocytes as evidence of inflammation.
Ileum: Villi oedema was occasionally noted in the various untreated and treated groups, but not in controls. The three C. concisus-positive mice in experiment 1 had no signs of inflammation of the ileum.
Colon: Two mice in the untreated group infected with the type ATCC 33237 strain, showed evidence of inflammation and lymphocyte infiltration.
Loss of body weight: Compared to controls, the mice infected with the C. concisus strains RH4204.98 and RH 10776.98 showed a significant weight loss (P < 0.05), while ATCC 33237-infected mice showed a similar trend, however, this was only borderline significant (P = 0.08) (experiment 3). The effect was clear from day 2 but wore off on day 5. At the end of the first week, all three groups of C. concisus-infected mice showed a net gain less than one g of bodyweight. The controls had a slowly progressive net weight gain of two g. These results are summarized in Table 2. After the first week no differences in weight were observed between the groups.
Loose stools: On day 2 and 3 faecal pellets in C. concisus-inoculated groups were loose and slimy compared to control groups (experiment 3).
Mortality: Two mice in experiment 2 died after the second C. concisus inoculation with the clinical strains RH4204.98 and RH5097.98. One mouse inoculated with the clinical isolate RH10776.98 died on day 5 (experiment 3).
The present model mimics a relevant intragastric exposure to C. concisus infection in immunocompetent BALB/cA mice upon cyclophosphamide and vancomycin pretreatment. Taken together, the results indicate a transient colonization of liver and ileum, with clinical signs of illness seen as loss of body weight and loose stools, however, the lack of consistent C. concisus isolation is troublesome in the present model.
The reason for these discrepancies is not clear, but may predominately arise from (1) difficulties in isolation of C. concisus and (2) lack of tissue samples from sick animals. The problem encountered in the isolation of C. concisus from faecal pellets collected in the cages may have been due to swarming contamination of Proteus spp. on many of the blood agar plates, which obstructed identification of other bacteria including C. concisus. Using the filter technique reduced this problem but did not alleviate this swarming. A method to separate faecal pellets from urine might increase the isolation rate of C. concisus in future studies. In addition, the inoculation dose (0.3 × 109 CFU) may have limited the chance of persistent colonization and development of full scale illness, as many researchers report doses above 1010 CFU before the successful recovery of bacteria is achieved[20,21]. PCR detection was poor and refined techniques must be used, which could also solve the problem of swarming. In the clinical parameters assessed, loss of body weight and loose stools were most prominent on the first three days of infection; after that time the effects of colonization seemed to wear off. As the first mice were sacrificed on day 7, no symptomatic animals were sacrificed, leaving the cultivation of C. concisus and histopathological studies to determine the presence of inflammation only in asymptomatic mice.
The fact that C. concisus was isolated from the liver of infected animals is consistent with previous studies of C. jejuni extraintestinal manifestations[28-30], where histopathological lesions have been described, as opposed to the present study. There have been occasional reports of humans developing hepatitis due to C. jejuni infections[31,32]. In our study of 98 patients with C. concisus infection[5] none had hepatitis, although several had disturbed liver biochemistry. The presence of Campylobacter spp. in liver is an intriguing finding that merits further investigation.
Overall inflammatory changes in the present study were scarce and limited to a total of three mice. The absence of substantial histopathological findings could be due to localised patches of colonization in the GI-tract, or that the damaging effects of colonization were small and resolved quickly before the mice were sacrificed seven days post-inoculation. Some studies have reported similar findings of discrepancies between colonization and the absence of lesions on histological examination[23], whilst studies of infections on limited-flora SCID mice present with severe histopathological lesions when symptomatically sick mice are sacrificed[24]. Similar results were found in human studies, as histopathological evidence of intestinal damage is limited to sick patients and not found in asymptomatically colonized patients[33].
The present panel of six C. concisus strains (Table 1) showed only minor differences in clinical outcome, but the only isolated strain after inoculation was the clinical strain RH10776.98, originally from an otherwise healthy traveller with diarrhoea. We have previously shown clinical differences when applying protein profiles as well as DNA-based typing systems to differentiate clinical C. concisus strains[5,11,25]. The clinical strain RH10776.98 belonged to the protein profile group 2 and genomospecies 2, both of which consisted of predominately immunocompetent patients complaining of diarrhoeal disease. However, clinical differences were not apparent as the type ATCC strain of protein group 1 and genomospecies 1 was also able to induce a near significant and comparable weight loss. The fact that genetic differences exist and that many Campylobacter spp. exhibit interstrain differences in colonization has been shown previously[34,35]. However, more strains need to be tested to clarify the question of strain variability in C. concisus infection.
Recently, a membrane bound haemolytic phos-pholipase has been identified in clinical C. concisus strains[7]. Also cytotoxic activity mimicking the Cytolethal Distending Toxin (CDT) has been found in the majority of tested C. concisus strains[8]. Taken together these findings suggest that a pathogenic role exists for at least certain subtypes of C. concisus strains. It is conceivable that C. concisus strains may differ in their pathogenicity due to the heterogeneity of the species[10-12,36].
Intragastric exposure to C. concisus infection in im-munocompetent BALB/cA mice upon cyclophosphamide and vancomycin pretreatment resulted in transient colonization of liver, jejunum and ileum in a few mice. The symptoms were loss of body weight and loose stools. As most mice were not diseased, other strains of mice and increased dose levels of inoculum should be studied with a focus on the first days after inoculation. Future studies should concentrate on the first three days of infection and symptomatic mice should be sacrificed for optimal detection of histopathological changes, as the organism is rapidly cleared from the GI-tract and symptoms resolve.
Our knowledge of the etiological agent in infectious diarrhoea can at present only account for some 50% of the cases presenting with gastroenteritis. Lately, Campylobacter concisus (C. concisus) has been associated with gastroenteritis in humans, however clear evidence of a pathological role is lacking. At present only few clinical laboratories worldwide perform an active search for C. concisus strains in routine sampling from diarrhoeal patients. The present study aims to determine if C. concisus can cause diarrhoeal illness in mice.
No study has addressed a pathogenic role of C. concisus in animal models. The demonstration of a pathogenic role of C. concisus in animals will fulfill Koch´s postulates and establish C. concisus as a pathogen to be considered in diarrhoeal disease. This study reports that transient colonization occurs and clinical symptoms are present in mice. However, the described model is not suitable for further research, as colonization is inconsistent and symptoms rapidly resolve.
This is the first study to report on C. concisus infection in mice.
This study proves that the colonization of mice GI tract and liver is possible and histopathological findings including microabscesses are present. Further studies using immunodeficient mice and focussing on the first days of infection are needed to justfully answer the question of pathogenicity. The liver pathology is an intriguing finding that merits attention in a human setting.
The experimental study is focused on establishment of experimental infection with Campylobacter concisus in mice. The experiments are in the field of intestinal microbiology. Experimental cohorts are well clustered; important Campylobacter strains are used. The manuscript is well written.
Peer reviewers: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Li JL L- Editor Webster JR E- Editor Lin YP
| 1. | Skirrow MB. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113-149. |
| 2. | Newell DG. Campylobacter concisus: an emerging pathogen? Eur J Gastroenterol Hepatol. 2005;17:1013-1014. |
| 3. | Lastovica AJ. Emerging Campylobacter spp: The tip of the iceberg. Clin Microbiol New. 2006;28:49-55. |
| 4. | Engberg J, On SL, Harrington CS, Gerner-Smidt P. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. J Clin Microbiol. 2000;38:286-291. |
| 5. | Aabenhus R, Permin H, On SL, Andersen LP. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis. 2002;34:248-252. |
| 6. | Maher M, Finnegan C, Collins E, Ward B, Carroll C, Cormican M. Evaluation of culture methods and a DNA probe-based PCR assay for detection of Campylobacter species in clinical specimens of feces. J Clin Microbiol. 2003;41:2980-2986. |
| 7. | Istivan TS, Coloe PJ, Fry BN, Ward P, Smith SC. Characterization of a haemolytic phospholipase A(2) activity in clinical isolates of Campylobacter concisus. J Med Microbiol. 2004;53:483-493. |
| 8. | Engberg J, Bang DD, Aabenhus R, Aarestrup FM, Fussing V, Gerner-Smidt P. Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteristics. Clin Microbiol Infect. 2005;11:288-295. |
| 9. | Van Etterijck R, Breynaert J, Revets H, Devreker T, Vandenplas Y, Vandamme P, Lauwers S. Isolation of Campylobacter concisus from feces of children with and without diarrhea. J Clin Microbiol. 1996;34:2304-2306. |
| 10. | Matsheka MI, Elisha BG, Lastovica AL, On SL. Genetic heterogeneity of Campylobacter concisus determined by pulsed field gel electrophoresis-based macrorestriction profiling. FEMS Microbiol Lett. 2002;211:17-22. |
| 11. | Aabenhus R, On SL, Siemer BL, Permin H, Andersen LP. Delineation of Campylobacter concisus genomospecies by amplified fragment length polymorphism analysis and correlation of results with clinical data. J Clin Microbiol. 2005;43:5091-5096. |
| 12. | Vandamme P, Falsen E, Pot B, Hoste B, Kersters K, De Ley J. Identification of EF group 22 campylobacters from gastroenteritis cases as Campylobacter concisus. J Clin Microbiol. 1989;27:1775-1781. |
| 13. | Fox JG, Ackerman JI, Taylor N, Claps M, Murphy JC. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am J Vet Res. 1987;48:85-90. |
| 14. | Babakhani FK, Bradley GA, Joens LA. Newborn piglet model for campylobacteriosis. Infect Immun. 1993;61:3466-3475. |
| 15. | Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol. 1988;54:2365-2370. |
| 16. | Russell RG, Blaser MJ, Sarmiento JI, Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989;57:1438-1444. |
| 17. | Newell DG. Animal models of Campylobacter jejuni colonization and disease and the lessons to be learned from similar Helicobacter pylori models. Symp Ser Soc Appl Microbiol. 2001;(30):57S-67S. |
| 18. | Berndtson E, Danielsson-Tham ML, Engvall A. Experimental colonization of mice with Campylobacter jejuni. Vet Microbiol. 1994;41:183-188. |
| 19. | Stanfield JT, McCardell BA, Madden JM. Campylobacter diarrhea in an adult mouse model. Microb Pathog. 1987;3:155-165. |
| 20. | Jesudason MV, Hentges DJ, Pongpech P. Colonization of mice by Campylobacter jejuni. Infect Immun. 1989;57:2279-2282. |
| 21. | Hodgson AE, McBride BW, Hudson MJ, Hall G, Leach SA. Experimental campylobacter infection and diarrhoea in immunodeficient mice. J Med Microbiol. 1998;47:799-809. |
| 22. | Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun. 2004;72:1116-1125. |
| 23. | Young VB, Dangler CA, Fox JG, Schauer DB. Chronic atrophic gastritis in SCID mice experimentally infected with Campylobacter fetus. Infect Immun. 2000;68:2110-2118. |
| 24. | Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006;74:5261-5271. |
| 25. | Aabenhus R, Permin H, Andersen LP. Characterization and subgrouping of Campylobacter concisus strains using protein profiles, conventional biochemical testing and antibiotic susceptibility. Eur J Gastroenterol Hepatol. 2005;17:1019-1024. |
| 26. | On SL. Identification methods for campylobacters, helicobacters, and related organisms. Clin Microbiol Rev. 1996;9:405-422. |
| 27. | Bastyns K, Chapelle S, Vandamme P, Goossens H, De Wachter R. Specific detection of Campylobacter concisus by PCR amplification of 23S rDNA areas. Mol Cell Probes. 1995;9:247-250. |
| 28. | Kita E, Oku D, Hamuro A, Nishikawa F, Emoto M, Yagyu Y, Katsui N, Kashiba S. Hepatotoxic activity of Campylobacter jejuni. J Med Microbiol. 1990;33:171-182. |
| 29. | Kita E, Nishikawa F, Kamikaidou N, Nakano A, Katsui N, Kashiba S. Mononuclear cell response in the liver of mice infected with hepatotoxigenic Campylobacter jejuni. J Med Microbiol. 1992;37:326-231. |
| 30. | Vuckovic D, Abram M, Doric M. Primary Campylobacter jejuni infection in different mice strains. Microb Pathog. 1998;24:263-268. |
| 31. | Reddy KR, Farnum JB, Thomas E. Acute hepatitis associated with campylobacter colitis. J Clin Gastroenterol. 1983;5:259-262. |
| 33. | Black RE, Perlman D, Clements ML, Levine MM, Blaser MJ. Human Volunteer Studies with Campylobacter jejuni. Campylobacter jejuni: Current status and future trends. Washington DC: American Society for Microbiology 1992; 207-215. |
| 34. | Carvalho AC, Ruiz-Palacios GM, Ramos-Cervantes P, Cervantes LE, Jiang X, Pickering LK. Molecular characterization of invasive and noninvasive Campylobacter jejuni and Campylobacter coli isolates. J Clin Microbiol. 2001;39:1353-1359. |
| 35. | Ahmed IH, Manning G, Wassenaar TM, Cawthraw S, Newell DG. Identification of genetic differences between two Campylobacter jejuni strains with different colonization potentials. Microbiology. 2002;148:1203-1212. |
| 36. | On SL, Harrington CS. Identification of taxonomic and epidemiological relationships among Campylobacter species by numerical analysis of AFLP profiles. FEMS Microbiol Lett. 2000;193:161-169. |