Published online Nov 21, 2008. doi: 10.3748/wjg.14.6655
Revised: October 31, 2008
Accepted: November 7, 2008
Published online: November 21, 2008
AIM: To investigate the innate immune reactivity of tumor necrosis factor-alpha (TNF-α), Toll-like receptor 4 (TLR4), and CD14 in the liver of non-alcoholic steatohepatitis (NASH) model rats.
METHODS: Male F344 rats were fed a choline-deficient L-amino-acid-defined (CDAA) diet. The rats were killed after 4 or 8 wk of the diet, and their livers were removed for immunohistochemical investigation and RNA extraction. The liver specimens were immunostained for TNF-α, TLR4, and CD14. The gene expressions of TNF-α, TLR4, and CD14 were determined by reverse-transcriptase polymerase chain reaction (RT-PCR). Kupffer cells were isolated from the liver by Percoll gradient centrifugation, and were then cultured to measure TNF-α production.
RESULTS: The serum and liver levels of TNF-α in the CDAA-fed rats increased significantly as compared with the control group, as did the immunohistochemical values and gene expressions of TNF-α, TLR4, and CD14 with the progression of steatohepatitis. TNF-α production from the isolated Kupffer cells of the CDAA-fed rats was elevated by lipopolysaccharide stimulation.
CONCLUSION: The expressions of TNF-α, TLR4, and CD14 increased in the NASH model, suggesting that TLR4 and CD14-mediated endotoxin liver damage may also occur in NASH.
- Citation: Kawaratani H, Tsujimoto T, Kitazawa T, Kitade M, Yoshiji H, Uemura M, Fukui H. Innate immune reactivity of the liver in rats fed a choline-deficient L-amino-acid-defined diet. World J Gastroenterol 2008; 14(43): 6655-6661
- URL: https://www.wjgnet.com/1007-9327/full/v14/i43/6655.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6655
| Target gene | Primer |
| TNF-α | 5’-TACTGAACTTCGGGGTGATTGGTCC-3’ (F) |
| 5’-CAGCCTTGTCCCTTGAAGAGAACC-3’ (R) | |
| TLR4 | 5’-CGCTTTCAGCTTTGCCTTCATTAC-3’ (F) |
| 5’-TGCTACTTCCTTGTGCCCTGTGAG-3’ (R) | |
| CD14 | 5’-GTGCTCCTGCCCAGTGAAAGAT-3’ (F) |
| 5’-GATCTGTCTGACAACCCTGAGT-3’ (R) | |
| β-actin | 5’-ACCACAGCTGAGAGGGAAATCG-3’ (F) |
| 5’-AGAGGTCTTTACGGATGTCAAAG-3’ (R) |
Some patients who are not regular drinkers of alcohol have hepatic histological features that mimic alcoholic hepatitis and can progress to liver cirrhosis[1]. This condition has been named non-alcoholic steatohepatitis (NASH) and is thought to begin with excessive fat accumulation in the liver (first hit), followed by aggravating factors such as oxidative stress, inflammatory cytokines, and endotoxins (second hit)[2].
Kupffer cells, the resident macrophages in the liver, are localized in the non-parenchymal tissue and involved in the organ’s innate immunity. Activation of Kupffer cells induces progression of NASH via tumor necrosis factor-alpha (TNF-α)[3]. The recent discovery of Toll-like receptor 4 (TLR4), a functional receptor on the surface of macrophages and various other types of cells that transmit Et signals, has led to recognition of molecules, including CD14, that bind to lipopolysaccharides (LPSs) as the mainstay of the mechanism that initiates signal transduction leading to LPS recognition and cellular activation[4].
NASH is characterized by hepatic steatosis, inflamm-ation, and fibrosis, with increased risk of developing cirrhosis and hepatocellular carcinoma (HCC)[5]. It is known that the choline-deficient L-amino-acid-defined (CDAA) fed rat model shows fatty accumulation and fibrosis, and after 16 wk the liver shows cirrhotic changes and nodules appear like HCC[6]. This model is sometimes used as a hepatocarcinogenesis model. In this study, we used a CDAA diet to produce one of the experimental animal models of NASH[7], to investigate the innate immune reactivity of the liver in NASH.
Male F344 rats (6-wk-old, weighing 180-200 g) were purchased from Japan SLC (Hamamatsu, Japan), and fed a CDAA diet for up to 8 wk to induce NASH. At 1, 4, and 8 wk, the control rats (fed a standard rat chow), and the CDAA-fed rats were killed and bled from the inferior vena cava (IVC). Liver tissues were excised after perfusion with PBS into the portal vein. All procedures were approved by our institutional animal care committee and conducted in accordance with Nara Medical University Guidelines for the Care and Use of Laboratory Animals.
Serum samples from the control and CDAA-fed rats were used to measure the serum ALT levels. The serum ALT levels were determined using a 7170 Clinical Analyzer (Hitachi High-Technologies, Tokyo, Japan).
Serum samples (50 μL) from the control and CDAA-fed rats were used to measure the TNF-α concentration by enzyme-linked immunosorbent assay (ELISA) kit (BioSource International, Camarillo, CA, USA).
Liver tissues were frozen in liquid nitrogen, powdered, and 30 mg were homogenized in 1 mL of lysis buffer. The supernatant (50 μL) was used to measure the TNF-α levels by ELISA kit (BioSource International).
The liver tissues were fixed in 40 g/L formalin, embedded in paraffin, and 5-μm thick sections were cut from the paraffin blocks for staining with hematoxylin-eosin (HE) and Azan stains. Histological grading and staging were performed using a modified scoring system based on the classification of either Matteoni et al[8] or Brunt et al[9] proposed NAFLD types 1-4 based on long-term outcome studies, while Brunt et al[9] proposed a system of grading and staging for NASH that follows methods of separate assessment for necroinflammatory lesions (grading) and fibrosis (staging) accepted in other forms of non-biliary chronic liver diseases.
The TNF-α immunohistochemical staining was performed on the paraffin sections using an anti-rat TNF-α/TNFSF1A antibody (R&D Systems, Minneapolis, MN, USA), and the TLR4 and CD14 immunohistochemical staining was performed on frozen sections using an anti-rat TLR4 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and an anti-rat CD14 antibody (Santa Cruz Biotechnology Inc.), respectively. After that, a Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA, USA), and DAB peroxidase substrate solution (Vector Laboratories Inc.), with counterstaining by Hematoxylin Mayer were used. The stained areas were analyzed by NIH image software (Version 1.61, U.S. National Institute of Health, Bethesda, MD, USA).
Kupffer cells were isolated from the control and CDAA-fed rat livers according to a previously described procedure[10,11]. In brief, the liver was perfused through the portal vein with 100 mL of PBS containing 0.5 mmol/L EGTA (Research Organics, Cleveland, OH, USA) solution. The perfusion was carried out at 37°C at a flow rate of 10 mL/min. Subsequent perfusion with Hanks’ balanced salt solution containing 0.5 g/L collagenase type IV (Wako, Osaka, Japan) (collagenase solution) was carried out at 37°C at a flow rate of 10 mL/min for 20 min. Then, the liver was excised and minced in the collagenase solution.
The liver slurry was filtered through gauze mesh, then washed with collagenase solution. Next, Percoll (Sigma-Aldrich Corp, St. Louis, MO, USA) was used for density gradient centrifugation of cells. Kupffer cells were collected from the layer between 1.04 and 1.06. They were then cultured in RPMI 1640 supplemented with 10% fetal bovine serum and SAG (50 μg/mL streptomycin, 50 μg/mL ampicillin, 0.3 g/L L-glutamine). The viability of the cultured Kupffer cells was determined by trypan blue exclusion. Kupffer cells were seeded at a density of 5 × 105 cells/mL on a 12-well plate with RPMI 1640. For LPS-stimulated group, Kupffer cells were then exposed to 1 μg/mL of LPS (Sigma-Aldrich Corp.) and incubated for 4.5 h at 37°C in a 50 mL/L CO2-humidified air atmosphere. The cells untreated with LPS were used as control. The supernatant was analyzed by ELISA kit (BioSource International) to measure the TNF-α concentration.
The total RNA was extracted from the powdered frozen liver samples using an RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Briefly, 2 μg of the RNA sample were used for cDNA synthesis using Moloney Murine Leukemia Virus (M-MLV), Reverse Transcriptase RNaseH Minus (Toyobo, Osaka, Japan), and RNase Inhibitor (Toyobo) to investigate the expressions of TNF-α mRNA, TLR4 mRNA, and CD14 mRNA. PCR procedure was performed as follows: the samples were heated at 94°C, subjected to 30 cycles of 45 s at 94°C for denaturing, 45 s at 60°C for annealing, and 2 min at 72°C for extension. Following RT-PCR, 10-μL samples of the amplified products were resolved by electrophoresis on 15 g/L agarose gel, and stained with ethidium bromide. Each PCR product was analyzed by NIH image software. The specific primers for TNF-α, TLR4, CD14, and β-actin used are described in Table 1.
All results are expressed as mean ± SD. The analysis was carried out using Statview software (version 5.0, SAS Institute, Cary, NC, USA). Statistical differences between groups were evaluated using the paired t-test. P < 0.05 was considered statistically significant.
The mean serum ALT level in the control rats was 41.7 ± 7.4 IU/L, as compared with 524.6 ± 101.7 IU/L, 267.9 ± 47.5 IU/L, and 251.4 ± 81.6 IU/L in the 1-, 4-, and 8-wk CDAA-fed rats, respectively. All CDAA-fed groups showed a statistically significant elevation in the serum ALT level as compared with the control group (P < 0.01).
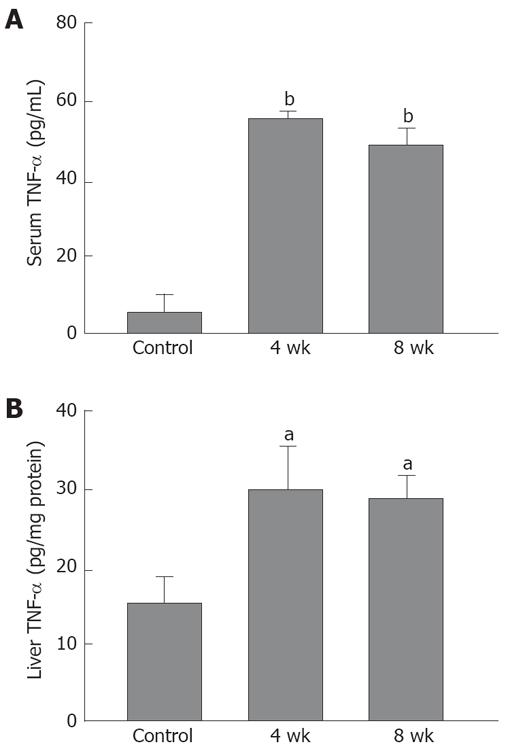
As compared with the control rats (5.2 ± 4.4 pg/mL), the serum TNF-α level in the 4-wk CDAA-fed rats was elevated (55.9 ± 1.7 pg/mL, P < 0.01), and that in the 8-wk CDAA-fed rats (48.2 ± 5.3 pg/mL, P < 0.01) was elevated (Figure 1A).
The liver TNF-α was 15.8 ± 3.6 pg/μg protein in the control rats, 30.3 ± 5.4 pg/μg protein in the 4-wk CDAA-fed rats, and 29.6 ± 4.4 pg/μg protein in the 8-wk CDAA-fed rats. The CDAA-fed groups showed a statistically significant elevation in the liver TNF-α level as compared with the control group (P < 0.05) (Figure 1B).
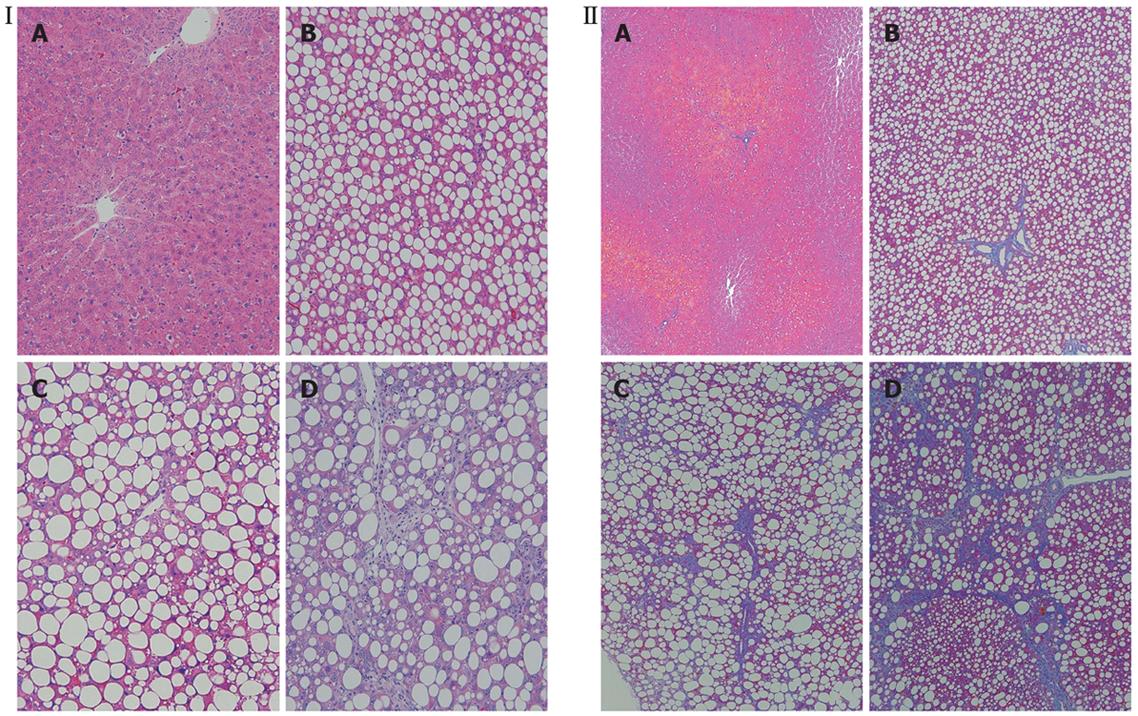
Histologically, the livers of the 1-wk CDAA-fed rats showed inflammation and fat deposits, but no fibrosis, and were classified as Matteoni’s type 2. The livers of the 4-wk CDAA-fed rats showed more inflammation, fat deposits, and fibrosis, equivalent to Matteoni’s type 3 and grade 2/stage 2 of Brunt’s NASH classification. The histological findings in the livers of the 8-wk CDAA-fed rats were equivalent to Matteoni’s type 4 and Brunt’s grade 2/stage 3 (Figure 2).
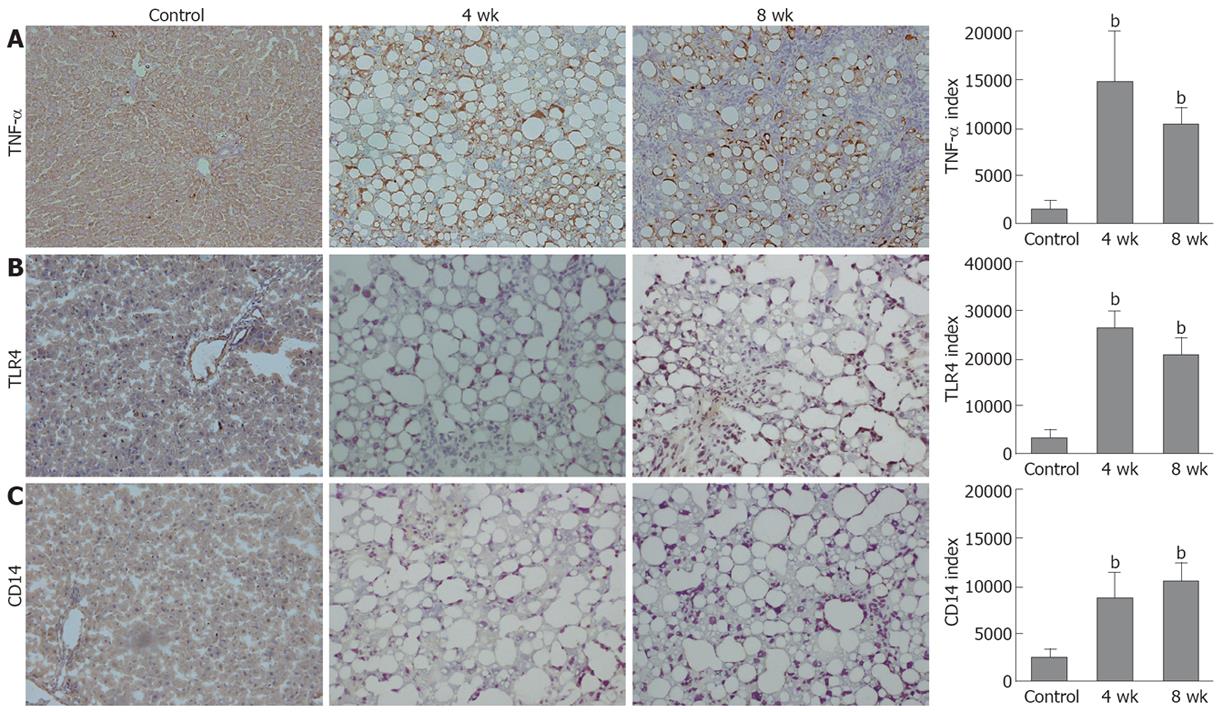
The TNF-α immunoreactivities in the tissue samples from the livers of the 4- and 8-wk CDAA-fed rats were increased as compared with those in the control rats. The TLR4 and CD14 immunohistochemical findings in the 4- and 8-wk CDAA-fed rats were also increased as compared with those in the control rats. Moreover, the immunopositive areas in those animals were localized either in the periphery of fatty spots or non-parenchymal cells. The semiquantitative analysis of TNF-α, TLR4, and CD14 staining revealed significantly increased immunoreactivity in the livers of the 4-wk (14600 ± 5700, 26400 ± 2300, 17400 ± 6000, respectively) and 8-wk (10600 ± 1800, 21600 ± 4700, 21300 ± 3900, respectively) CDAA-fed rats as compared with the control rats (1560 ± 1080, 3160 ± 1000, 4290 ± 1110, respectively) (P < 0.01) (Figure 3).
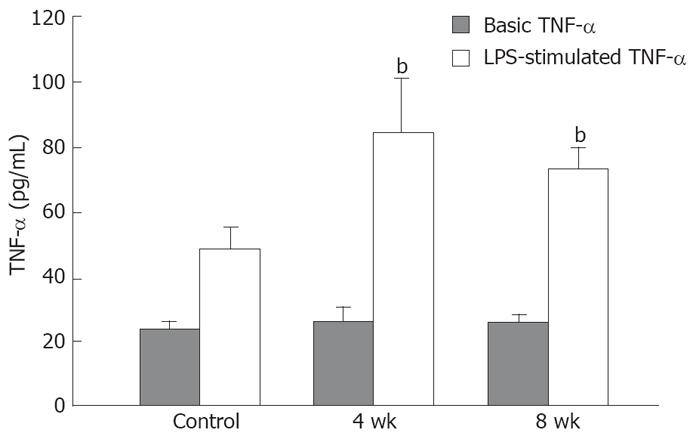
The viability of the cultured Kupffer cells was over 98% as determined by trypan blue exclusion. The basic TNF-α production of the Kupffer cells isolated from 4- (26.4 ± 2.3 pg/mL) and 8-wk CDAA-fed rats (26.6 ± 1.4 pg/mL) was equal to that of the control rats (23.4 ± 1.8 pg/mL). After LPS stimulation, the TNF-α production in the control rats (48.9 ± 7.5 pg/mL) was significantly higher than the basic control levels, and that in the 4-wk (85.8 ± 16.8 pg/mL) and 8-wk (73.8 ± 8.4 pg/mL) CDAA-fed rats was significantly higher than in the control rats (P < 0.01) (Figure 4).
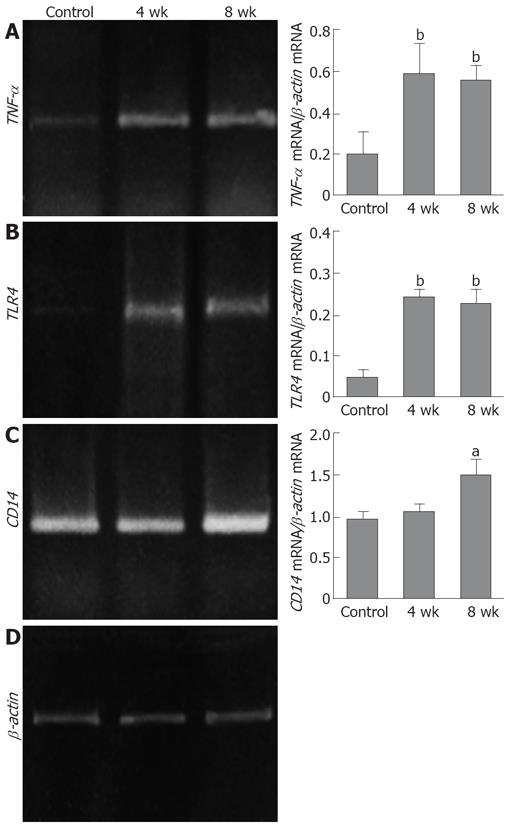
The expression of both TNF-α mRNA and TLR4 mRNA was significantly increased in the livers of the 4- and 8-wk CDAA-fed rats as compared with those in the control group (P < 0.01). The expression of CD14 mRNA was increased in the livers of the 8-wk CDAA-fed rats as compared with that in the control group (P < 0.05) (Figure 5).
Kupffer cells secrete inflammatory cytokines[12] and reactive oxide[13], which affect the hepatocytes, stellate cells, endothelial cells, etc[14]. In alcoholic hepatitis, inflammatory cytokines, such as TNF-α, induce the liver injury[15]. After chronic ethanol feeding, Kupffer cells exhibit enhanced sensitivity to LPS-stimulated TNF-α production[16]. Increased serum levels of TNF-α have been noticed in patients with NASH[17]. The mechanism of the latter may be secretion from adipocytes, as a result of obesity, or secretion from Kupffer cells activated by endogenous endotoxin originating from enteric bacteria. The activated Kupffer cells induce increases in the levels of cytokines, such as TNF-α or reactive oxide, which may be involved in the progression of NASH. Rats fed a high-fat diet become obese and show insulin resistance. In addition, the activities of both nuclear factor kappa enhancer binding protein (NF-κB) and the inhibitor of NF-κB kinase beta (IKKβ) significantly increase in the liver, and the expression of inflammatory cytokines such as TNF-α becomes excessive[18]. Our results showed that the livers of CDAA-fed rats developed inflammation in the early stage, and this was sustained by chronic feeding of the diet. After 4 wk or more, the livers of the CDAA-fed rats developed progressive steatohepatitis. In the present study, we confirmed by ELISA, immunohistochemistry, and RT-PCR that both the serum level of TNF-α and the liver TNF-α expression were increased, suggesting involvement of TNF-α in the development and progression of NASH similar to that in alcoholic hepatitis. Moreover, the TNF-α production of the isolated Kupffer cells was equal in the NASH model. Kupffer cells from the control rats were sensitive to LPS stimulation. Furthermore, those from NASH were more sensitive to LPS stimulation, suggesting that the continuous inflammation leads to up-regulation of the LPS-stimulated TNF-α production by the activated Kupffer cells.
To date, 13 TLRs have been described in mice and 11 in humans. Recently, TLR4 has been highlighted as an important membrane protein[19,20] and is now established as the receptor for LPS. CD14 binds LPS and subsequently presents it to TLR4 and MD-2, which activate the intracellular signaling pathway via myeloid differentiation factor 88 (MyD 88), resulting in NF-κB activation[21]. Experiments using TLR4 knockout (KO) mice or CD14 KO mice have revealed a reduced reaction to LPS and a reduced expression of TNF-α[22,23], indicating that LPS signaling induces TNF-α production via TLR4 or CD14. The intake of alcohol facilitates the permeability of endotoxin from the gut to the portal vein, and the increased concentration of endotoxin in the portal vein leads to Kupffer cell activation via TLR4, with consequent increases in the liver expression of TNF-α, progression of inflammation, and fibrosis of the liver[24]. In the experimental chronic liver injury model, the expression of TLR4 in isolated Kupffer cells increases with progression of liver injury[25], suggesting a correlation between inflammation and expression of TLR4. The present study showed increased expression of mRNA levels and immunohistochemical values of TNF-α, TLR4, and CD14 in the livers of rats with NASH induced by CDAA diet, suggesting that TLR4-mediated endotoxin liver damage may also occur in NASH. Rivera et al[26] also demonstrated enhanced TLR4 expression together with steatohepatitis in the liver of mice fed a methionine choline-deficient (MCD) diet. They further reported that the liver injury was attenuated in the TLR4 mutant mice and in Kupffer cell-depleted mice, and pointed out the importance of TLR4 signaling and Kupffer cells in the pathogenesis of steatohepatitis. Although these models lack obesity and insulin resistance, two major characteristics of NASH in humans, the possible relation of Kupffer cells with inflammation, steatosis, and fibrosis may resemble the human NASH. We also demonstrated decreased Kupffer cell phagocytic activity in this model[27]. The possible Kupffer cell dysfunction characterized by decreased phagocytic activity and increased cytokine production should be investigated in future studies.
Although the pathogenic mechanism of NASH is still unclear, its histopathology is similar to that of alcoholic hepatitis. Enhanced activation of the TLR4 dependent signaling pathways was also observed after chronic ethanol feeding[28]. There is a possibility that TLR4-dependent innate immunity induced by intestinal bacterial endotoxin may work as a common pathway in these two types of steatohepatitis. Further studies are needed to confirm this hypothesis.
In conclusion, our study focused on the innate immune reactivity of livers in the rat model of NASH, and we found overexpressions of TNF-α, TLR4, and CD14 in association with liver fibrosis and inflammation. These results suggest that TLR4 and CD14-mediated endotoxin liver damage may also occur in NASH.
Recently, non-alcoholic steatohepatitis (NASH) has become a common disease in Japan. The two-hit theory has been widely accepted to explain the progression from simple steatosis to NASH. Moreover, NASH is known as a disease progressing to liver cirrhosis or hepatocellular carcinoma (HCC). The mechanism of NASH progression has not been clarified yet. Authors investigated the engagement of Toll-like receptor 4 (TLR4) or CD14 in the rat NASH model.
This is the first report showing the innate immune response in the rat NASH model. Authors found overexpression of tumor necrosis factor-alpha (TNF-α), TLR4, and CD14 in association with liver fibrosis and inflammation. These results suggest that TLR4 and CD14-mediated endotoxin liver damage may occur in NASH.
The present study demonstrated the overexpression of TNF-α, TLR4, and CD14 in the rat NASH model. This result suggests that the involvement of endotoxin-induced TLR4 activation led to the NASH progression.
The present study is an experimental research using the rat NASH model, which was made by feeding a choline-deficient L-amino-acid-defined diet to rats, to examine their innate immunity. For understanding NASH progression, further evaluation of the cytokine production from the Kupffer cells and the expressions of TLR4 and CD14 from the isolated Kupffer cells should be employed in the subsequent experiments.
TLR4 is a transmembrane receptor for lipopolysaccharides (LPS) on the surface of macrophages. LPS stimulation activates TLR4 by binding with CD14, resulting in pro-inflammatory cytokine production, like TNF-α.
Authors described the importance of innate immune responses to the pathogenesis of rat model of NASH. The findings are important.
Peer reviewer: Satoshi Yamagiwa, MD, PhD, Division of Gastroentero-logy and Hepatology, Niigata University Graduate School of Medical and Dental Sciences, 757 Asahimachi-dori 1, Chuo-ku, Niigata 951-8510, Japan
S- Editor Li DL L- Editor Logan S E- Editor Lin YP
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. |
| 2. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. |
| 3. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. |
| 4. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. |
| 5. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. |
| 6. | Sakaida I, Kubota M, Kayano K, Takenaka K, Mori K, Okita K. Prevention of fibrosis reduces enzyme-altered lesions in the rat liver. Carcinogenesis. 1994;15:2201-2206. |
| 7. | Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89-104. |
| 8. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. |
| 9. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. |
| 10. | Olynyk JK, Clarke SL. Isolation and primary culture of rat Kupffer cells. J Gastroenterol Hepatol. 1998;13:842-845. |
| 11. | Seglen PO. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82:391-398. |
| 12. | Tsujimoto T, Kuriyama S, Yamazaki M, Nakatani Y, Okuda H, Yoshiji H, Fukui H. Augmented hepatocellular carcinoma progression and depressed Kupffer cell activity in rat cirrhotic livers. Int J Oncol. 2001;18:41-47. |
| 13. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. |
| 14. | Diehl AM. Recent events in alcoholic liver disease V. effects of ethanol on liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1-G6. |
| 15. | Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461-474. |
| 16. | Aldred A, Nagy LE. Ethanol dissociates hormone-stimulated cAMP production from inhibition of TNF-alpha production in rat Kupffer cells. Am J Physiol. 1999;276:G98-G106. |
| 17. | Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. |
| 18. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. |
| 19. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. |
| 20. | Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689-10692. |
| 21. | Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. |
| 22. | Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407-414. |
| 23. | Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-3752. |
| 24. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. |
| 25. | Hua J, Qiu de K, Li JQ, Li EL, Chen XY, Peng YS. Expression of Toll-like receptor 4 in rat liver during the course of carbon tetrachloride-induced liver injury. J Gastroenterol Hepatol. 2007;22:862-869. |
| 26. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. |
| 27. | Tsujimoto T, Kawaratani H, Kitazawa T, Hirai T, Ohishi H, Kitade M, Yoshiji H, Uemura M, Fukui H. Decreased phagocytic activity of Kupffer cells in a rat nonalcoholic steatohepatitis model. World J Gastroenterol. 2008;14:6036-6043. |
| 28. | Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood). 2003;228:882-890. |