Published online Nov 14, 2008. doi: 10.3748/wjg.14.6575
Revised: September 11, 2008
Accepted: September 18, 2008
Published online: November 14, 2008
Here we report a new case of clear cell adenocarcinoma (CCA) of the colon in a 54-year-old Caucasian man. Despite of the previous reported cases, the lesion was located in the right colon and was not associated with the conventional adenoma. We performed immunohistochemical and molecular analyses in order to explore whether the CCA had the molecular features generally associated with conventional colorectal carcinoma. The immunohistochemical and molecular analyses showed that the different morphology of CCA does not reflect a distinct biological entity but only an unusual morphological variant of intestinal carcinoma.
- Citation: Barisella M, Lampis A, Perrone F, Carbone A. Clear cell adenocarcinoma of the colon is a unique morphological variant of intestinal carcinoma: Case report with molecular analysis. World J Gastroenterol 2008; 14(42): 6575-6577
- URL: https://www.wjgnet.com/1007-9327/full/v14/i42/6575.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6575
Clear cell adenocarcinomas (CCAs) are very rare in the colon[1-3]. They generally affect elderly men, are preferentially located in the left colon and almost all form part of a larger conventional adenoma[4,5].
It is not known whether they are biologically different from morphologically conventional colorectal adenocarcinomas[6-9].
Here, an intriguing variant of CCA, unusual in term of location and morphology is described. Moreover, we have evaluated some pathways reported as deregulated in the conventional colorectal adenocarcinoma[10]. To this, we performed an extensive immunohistochemical analysis and for the first time a molecular analysis, including the genomic sequencing of KRAS gene and the main deregulated genes belonging to the Wnt pathway such as APC and β-catenin.
In September, 2000, a 54-year-old Caucasian man with a family history of gastrointestinal cancer and a personal 7-year history of multiple colon polyps, came to our Institution for a routine annual control colonoscopy. On this occasion 14 flat lesions with a diameter of 5-15 mm were found extending throughout the large intestine, some of which were endoscopically removed and histologically analysed, including one located in the left colon that was histologically a tubulo-villous adenoma with extensive clear cell aspects. The patient underwent subsequent colonoscopies with polyp resections in 2001, 2002, 2003, 2004 and 2005. All of these lesions were tubular adenomas. At the time of the last control colonoscopy in September 2005, a flat 0.9 cm lesion of the hepatic flexure was endoscopically revealed and biopsied, and was found to be a high-grade dysplastic adenoma with extensive clear cell features. The same lesion was re-biopsied in 2006 and 2007 with the same result. No conventional adenoma was identified. After undergoing total colectomy in June, 2007, the patient now feels well.
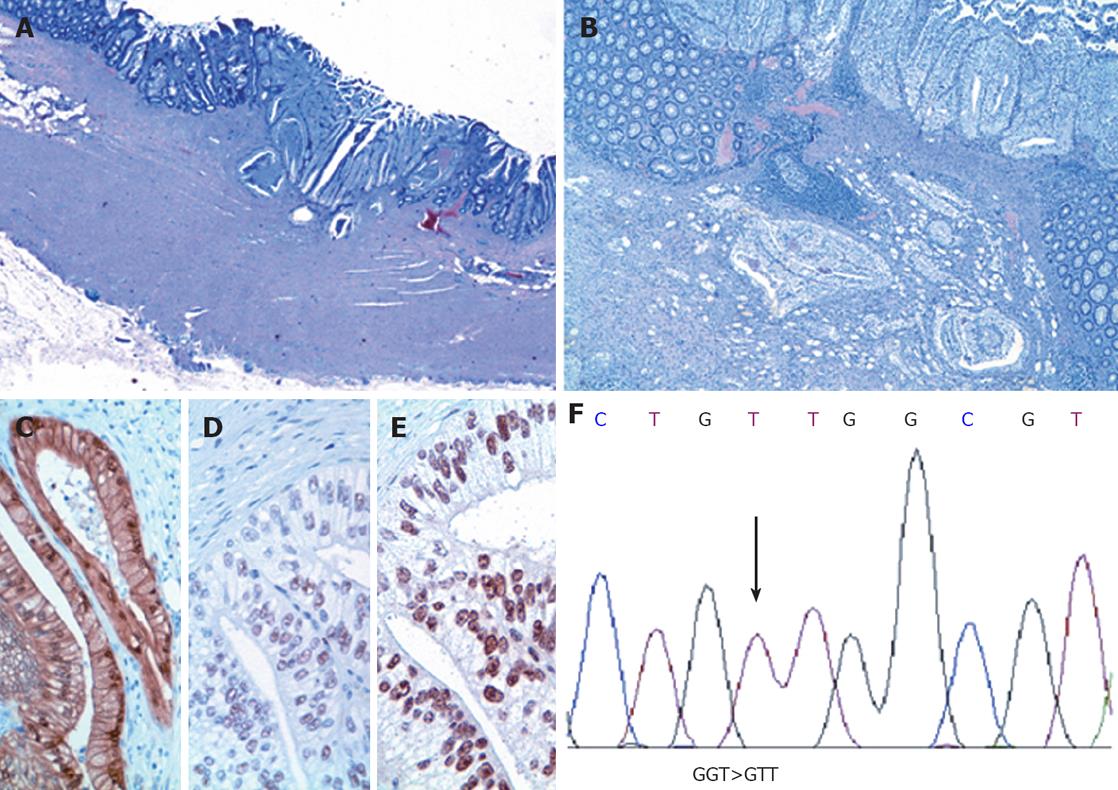
The gross specimen was a total colectomy of 115 cm with the flat, previously biopsied 0.9 cm lesion at the hepatic flexure and other pedunculated lesions in the rest of the colon. Microscopic examination showed that the pedunculated lesions were multiple tubular adenomas, and the 0.9 cm lesion of the hepatic flexure was a CCA invading the muscularis propria (Figure 1A). The CCA had a solid growth at the surface and a tendency to grow as single cells at the periphery (cellular budding); there was neither intratumoral inflammatory infiltration nor vascular invasion and no residual classic adenoma at the periphery (Figure 1B). Nodes were negative.
Five-micrometer thick, formalin-fixed (10% buffered formalin), paraffin-embedded tissue sections were immunohistochemically studied for CK20 (mouse KS20.8, BiOptica; 1:100; 6 min 95°C heated in 0.01 mol/L citrate buffer, pH 6.0), CEA polyclonal antibody (rabbit poly, DAKO; 1:4000; 0.1% trypsin 15 min), CK7 (clone K72.7, NeoMarkers; 1:400; 0.1% trypsin 15 min), alpha-feto protein (rabbit poly, DAKO; 1:2000; 0.1% trypsin 15 min), CD 10 (mouse 56C6, Neo Markers, 1:20, 6 min 95°C heated in 0.1 mol/L citrate buffer, pH6.0), vimentin (clone V9, DAKO; 1:400; 6 min 95°C heated in 0.1 mol/L citrate buffer, pH 6.0), β-catenin (clone 14, Transduction; 1:4000; 6 min 95°C heated in 0.1 mol/L citrate buffer, pH 6.0), hMLH1 (G168-15, Santa Cruz; 1:10; 2 min 120°C heated in 0.1 mol/L citrate buffer, pH 6.0), hMSH2 (NA27-100 μg, Oncogene; 1:40; 2 min 120°C heated in 0.1 mol/L citrate buffer pH 6.0) and p53 (clone DO7, Novocastra; 1:400; 6 min 95°C heated in 0.1 mol/L citrate buffer, pH 6.0). The positive controls were a sporadic aggressive fibromatosis sample with a known mutation in the CTNNB1 gene[11] for β-catenin; two colorectal cancer samples from hereditary non-polyposis colorectal cancer patients carrying MLH1 or MSH2 germline mutations for hMLH1 and hMSH2; a serous ovarian carcinoma with a known TP53 mutation for p53. The samples were strongly positive for CK20 and CEA polyclonal antibody, and negative for CK7, alpha-feto protein, CD10 and vimentin, thus supporting the intestinal origin of the CCA[12]. They also showed p53 focal nuclear positivity, strong β-catenin nuclear positivity in the glands (Figure 1C) and nuclear positivity for hMLH1 and hMSH2 (Figure 1D to E). The proliferation index was high (> 90%).
After genomic DNA extraction (QIAmp DNA mini Kit, Qiagen, Chatsworth, CA, USA), exon 1 of the KRAS gene was amplified by means of polymerase chain reaction (PCR) in order to seek potential mutations on the two foremost codons[12-13], which have been reported to be mutated in morphologically conventional colorectal carcinoma. Given the strong β-catenin nuclear positivity revealed by immunohistochemistry, exon 3 of the β-catenin gene was sequenced. The PCR amplifications were carried out using a standard protocol, and previously described primers and conditions[11,13]. In addition, specific primers were designed by means of Primer3 software to amplify codons 1368-1679 located in exon 15 of the APC gene and encompassing the mutation cluster region (MCR), where more than 60% of APC mutations have been detected in conventional colorectal cancer[14]. All of the PCR products were directly sequenced using an ABI Prism 377 (Applied Biosystems, Foster City, CA, USA) and evaluated by means of Sequence Navigator software (Applied Biosystems, Foster City, CA, USA). KRAS sequencing revealed the previously described point mutation GGT>GTT at codon 12, which leads to the activating aminoacid substitution Gly12Val (Figure 1F), whereas no mutations were found in the MCR of the APC gene or in the β-catenin gene.
Colon CCAs are rare, usually affect the left colon of elderly men, and are treated by means of polypectomy (in most cases) or segmental resection. Almost all CCAs form part of a larger conventional adenoma, thus supporting the hypothesis of a linear carcinogenetic sequence from conventional adenoma, to clear cell-type adenomas and CCAs.
However, our patient was a middle-aged man, the lesion was located in the right colon (hepatic flexure) and was not accompanied by a conventional adenoma, although many other tubular adenomas affected the whole colon, and the patient underwent total colectomy. Histologically, the CCA showed superficial solid growth and diffuse cellular budding at the periphery, but no vascular invasion. Immunohistochemistry ruled out other possible origin from extracolonic primary tumors, including renal clear cell carcinoma and carcinoma arising from the mullerian system.
We subsequently undertook immunohistochemical and molecular analyses in order to explore whether the CCA had the molecular features generally associated with conventional colorectal carcinoma. The positive nuclear immunostaining for hMLH1 and hMSH2 was consistent with microsatellite stability; the focal p53 nuclear immunoreactivity strongly suggested the absence of disabled p53 protein; and the β-catenin nuclear immunostaining was in line with activation of the Wnt pathway reported in conventional colorectal carcinoma[15]. However, neither APC nor β-catenin mutations were found, a result that we are inclined to attribute to the incomplete sequencing of APC exon 15 due to the very small amount of tumour tissue available.
However, molecular analysis of the KRAS gene by means of genomic DNA automatic sequencing revealed the activating KRAS mutation Gly12Val. This suggests that the analyzed CCA shares the KRAS genotype characterizing most (approximately 40%) conventional colorectal carcinomas.
The different morphology of CCA, therefore, does not seem to reflect a distinct biological entity, but an unusual morphological variant with similar molecular profile of conventional colorectal carcinoma.
Peer reviewer: Kevin J Spring, PhD, Conjoint Gastroenterology, RBWHF-CRC & QIMR, PO Royal Brisbane Hospital, Herston, Brisbane 4029, Australia
S- Editor Li DL E- Editor Yin DH
| 1. | Hamilton SR, Aaltonen LA (eds): World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press 2000; 110. |
| 3. | Soga K, Konishi H, Tatsumi N, Konishi C, Nakano K, Wakabayashi N, Mitsufuji S, Kataoka K, Okanoue T, Mukaisho K. Clear cell adenocarcinoma of the colon: a case report and review of literature. World J Gastroenterol. 2008;14:1137-1140. |
| 4. | Suzuki H, Ohta S, Tokuchi S, Moriya J, Fujioka Y, Nagashima K. Adenoma with clear cell change of the large intestine. J Surg Oncol. 1998;67:182-185. |
| 5. | Domoto H, Terahata S, Senoh A, Sato K, Aida S, Tamai S. Clear cell change in colorectal adenomas: its incidence and histological characteristics. Histopathology. 1999;34:250-256. |
| 6. | Hao LS, Zhu X, Zhao LH, Qian K, Zhou Y, Bu J, Wu XT. Clear cell adenocarcinoma of colorectum: a case report and review of the literature. Acta Gastroenterol Belg. 2007;70:235-238. |
| 7. | Ko YT, Baik SH, Kim SH, Min BS, Kim NK, Cho CH, Lee SK, Kim HG. Clear cell adenocarcinoma of the sigmoid colon. Int J Colorectal Dis. 2007;22:1543-1544. |
| 8. | Braumann C, Schwabe M, Ordemann J, Jacobi CA. The clear cell adenocarcinoma of the colon: case report and review of the literature. Int J Colorectal Dis. 2004;19:264-267. |
| 9. | Rubio CA. Clear cell adenocarcinoma of the colon. J Clin Pathol. 1995;48:1142-1144. |
| 10. | Frattini M, Balestra D, Suardi S, Oggionni M, Alberici P, Radice P, Costa A, Daidone MG, Leo E, Pilotti S. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res. 2004;10:4015-4021. |
| 11. | Signoroni S, Frattini M, Negri T, Pastore E, Tamborini E, Casieri P, Orsenigo M, Da Riva L, Radice P, Sala P. Cyclooxygenase-2 and platelet-derived growth factor receptors as potential targets in treating aggressive fibromatosis. Clin Cancer Res. 2007;13:5034-5040. |
| 12. | D'Amato A, Gentili V, Santella S, Pronio A, Montesani C. [Synchronous neoplasms of the colon and kidney: analysis of 2 case reports]. Chir Ital. 2000;52:83-86. |
| 13. | Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mondellini P, Bongarzone I, Collini P, Gariboldi M, Pilotti S. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436-7440. |
| 14. | Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721-733. |