Published online Nov 7, 2008. doi: 10.3748/wjg.14.6376
Revised: September 16, 2008
Accepted: September 23, 2008
Published online: November 7, 2008
AIM: To evaluate the relation of cluster of differentiation 44 (CD44) expression with clinicopathological features of gastric adenocarcinoma, and also its effect on prognosis with an emphasis on the differences between intestinal and diffuse types.
METHODS: From 2000 to 2006, 100 patients with gastric adenocarcinoma, who had undergone total or subtotal gastrectomy without any prior treatment, were studied. Haematoxylin & eosin (HE) staining was used for histological evaluation, including the type (Lauren’s classification) and grading of the tumor. The expression of CD44 in the gastric adenocarcinoma mucosa and the adjacent mucosa were determined by immunohistochemistry. The survival analysis was obtained using the Kaplan-Meier test.
RESULTS: Of 100 patients, 74 (74%) patients were male. The tumors were categorized as intestinal type (78%) or diffuse type (22%). Sixty-five percent of patients were CD44-positive. CD44 expression was not detected in normal gastric mucosa. Rather, CD44 was more commonly expressed in the intestinal subtype (P = 0.002). A significant relation was seen between the grade of tumor and the expression of CD44 (P = 0.014). The survival analysis showed a poor prognosis of patients with CD44-positive tumors (P = 0.008); and this was more prominent in the intestinal (P = 0.001) rather than diffuse type.
CONCLUSION: Cell adhesion molecule CD44 is highly expressed in gastric adenocarcinoma. CD44 expression is correlated with a poor prognosis in patients with the intestinal type of gastric adenocarcinoma. CD44 can, therefore, be utilized as a prognostic marker for this group of patients.
- Citation: Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani A, Moaven O, Bahrani M. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol 2008; 14(41): 6376-6381
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6376.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6376
| Data | Percentage (%) | |
| Sex | ||
| M | 74 | |
| F | 26 | |
| Age (yr) | ||
| < 65 | 47 | |
| > 65 | 53 | |
| Location | ||
| Cardia | 37 | |
| Body & Fundus | 33 | |
| Antrum | 30 | |
| Histology type | ||
| Intestinal | 78 | |
| Diffuse | 22 | |
| Grade | ||
| Well-Diff | 54 | |
| Mod-Diff | 17 | |
| Poor-Diff | 29 | |
| T | ||
| T1 | 0 | |
| T2 | 5 | |
| T3 | 84 | |
| T4 | 11 | |
| N | ||
| N0 | 16 | |
| N1 | 51 | |
| N2 | 30 | |
| N3 | 2 | |
| M | ||
| M0 | 98 | |
| M1 | 2 | |
| Stage | ||
| 1 | 3 | |
| 2 | 13 | |
| 3 | 71 | |
| 4 | 13 | |
Gastric malignancy is one of the leading types of cancer and is the second most common cause of cancer-related death in the world[1,2]. According to reports from Iran’s Ministry of Health, gastric cancer is the most common fatal gastrointestinal malignancy, while cancer is the third most common cause of mortality in this country[3,4]. Adenocarcinoma is the most prevalent type of gastric cancer. According to Lauren’s histological classification, it is subdivided into diffuse and intestinal pathologic subtypes, each having different epidemiological and prognostic features[5,6]. Multiple genetic and epigenetic alterations in oncogenes, tumor-suppressor genes, cell-cycle regulators, cell adhesion molecules and DNA repair genes are implicated in the multistep process of human stomach carcinogenesis[6,7], and different genetic pathways have been proposed for these two subtypes of gastric carcinoma[2]. Depth of invasion and lymph node metastasis result from the polygene, and their protein expression in gastric carcinoma. The key step of the basic and clinical research of gastric carcinoma is to discover the related etiological biomarkers.
The loss of normal cellular adhesion is a significant event in human cancer development. Metastasis is characterized by a loss of adhesion that allows cancer cells to invade, and leave the site of origin, subsequently adhering to other sites, such as lymph nodes, liver, or peritoneum[8]. Cluster of differentiation 44 (CD44) is a transmembrane glycoprotein involved in cellular adhesion. This polymorphic integral membrane glycoprotein, which is expressed by many cell types, serves as the principal transmembrane hyaluronate receptor. It is considered a determinant of metastatic and invasive behavior in different malignancies, such as lung carcinoma, malignant melanoma, leukemia, breast cancer, as well as gastrointestinal carcinomas[2,9,10]. On the other hand, contradictory reports concerning the biological role of CD44 in tumorigenesis and its clinical value in prognosis have also been presented[9]. The expression of CD44 potentiates tumor cells to adhere to the extracellular matrix through ligands such as hyaluronan and facilitates the efficient formation of cell colonies[11,12].
The reported frequency of CD44 expression in human gastric carcinoma specimens varies widely from 31% to 72%, most likely reflecting the differences in the study population[13,14]. The intensity of CD44 expression has been reported to correlate with an increased depth of invasion[13], although different correlations have been reported in the two histological types of this cancer.
In this study, the expression of CD44 in patients with gastric carcinoma was measured by immunohistochemical method, with an aim to evaluate the relation of CD44 expression with clinicopathological features and also its effect on prognosis with an emphasis on the differences between intestinal and diffuse types.
From 2000 through 2006, 100 gastric adenocarcinoma patients, who underwent total or sub-total gastrectomy without any prior treatment such as chemotherapy or radiation therapy at Omid Oncology Hospital (Mashhad, Iran), were enrolled in this study. All patients had been residing in the north-eastern provinces of Iran at the time of surgery. Demographic data of all patients were recorded and TNM staging was performed according to AJCC (American Joint Committee on Cancer) staging by supra- and infra-diaphragmatic imaging studies. Follow-up data were also gathered from patients, including local and distant recurrence and metastasis. The study protocol was approved by the Clinical Research Ethics Committee of the Mashhad University of Medical Sciences.
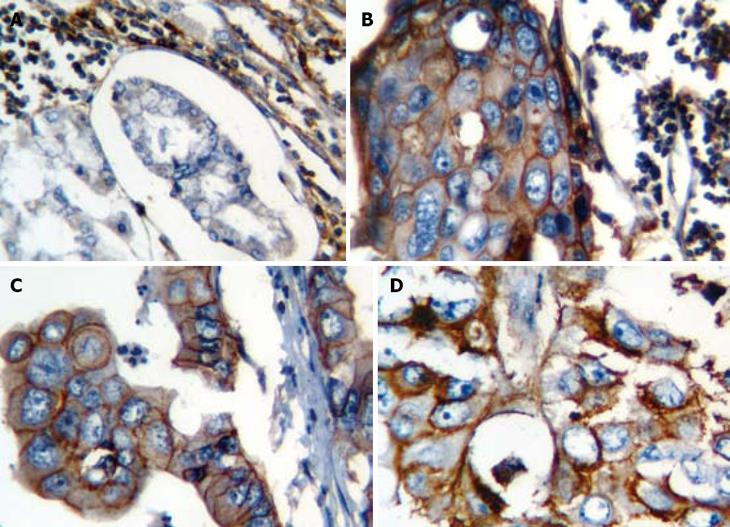
Formalin-fixed and paraffin wax-embedded gastric adenocarcinoma specimens from these patients were selected from the pathology archive. Specimen blocks were stained with HE and histological typing was determined according to the Lauren’s classification and tumor grade (well-differentiated, moderately-differentiated and poorly-differentiated). The pathologist reviewed all the blocks and chose one block with more tumoral tissue and less necrotic tissue for immunostaining. Specimens were cut into 4-μm thick sections, and the sections were dewaxed and processed for immunohistochemical (IHC) staining. The sections were stained using the streptavidin-biotin-peroxidase complex method (Dako LSAB2 system, Denmark). Mouse anti-CD44 monoclonal antibody (1:50 dilution; clone DF1485, Dakocytomation, Denmark) which is able to detect all isoforms of CD44, was employed as the primary antibody for 30 min. Internal lymphocytes were used as positive control, and normal gastric tissue as negative control. Also, for negative control, the primary antibody was omitted.
All sections were evaluated by the pathologist who was unaware of the clinical outcome of the patients. Tumors with more than 5% of CD44-positive cancer cells were regarded as positive. The results were reported as positive (cytoplasmic and/or membranous staining) or negative with the percentage of positive cells for each section.
The correlation between the CD44 expression status and clinicopathological variables was analyzed using parametric and non-parametric tests run by the statistical software SPSS version 13. Also a log-linear model by the statistical software SAS was used for more detailed analysis of the data. The survival analysis was performed by the Kaplan-Meier test. A P value less than 0.05 was considered statistically significant.
This study included 100 gastric adenocarcinoma cases (74 males and 26 females; male/female ratio: 2.8), with a mean age of 63.3 years (range 26-82 years). Data about stage, grade, histological types and location of tumors are summarized in Table 1. Forty-seven percent of patients were less than 65 years old. The mean age of patients with poorly differentiated tumors was significantly lower than other patients (55 years vs 65 years, P = 0.019). Stage IV disease was significantly more common among patients younger than 65 years (P = 0.02). All stageIcases were observed among patients older than 65 years. The diffuse-type cancer was two times higher among the patients younger than 65 years (P = 0.021).
Sixty-five percent of patients were CD44-positive and 35% were CD44-negative. CD44 staining was not detected in any adjacent normal gastric mucosa (Figure 1). Of 65 CD44-positive cases, 52 showed CD44 staining only on tumor cell surface membrane, while 13 showed both in cytoplasm and membrane.
In addition, a significant difference in CD44 expression was seen between the two histological subtypes: intestinal-type showed a significantly higher CD44 positivity (71%) compared to diffuse-type (42%) (P = 0.002). Moreover, poorly differentiated carcinomas showed a significantly less CD44 positivity, indicating a significant relation between CD44 expression, and the grade of tumor (P = 0.014; log linear, P = 0.004). Patients with stage 4 cancer expressed CD44 more than other stages, although this was not statistically significant. Other studied variables (sex, age and location of tumor) did not show any significant correlation with the expression of CD44.
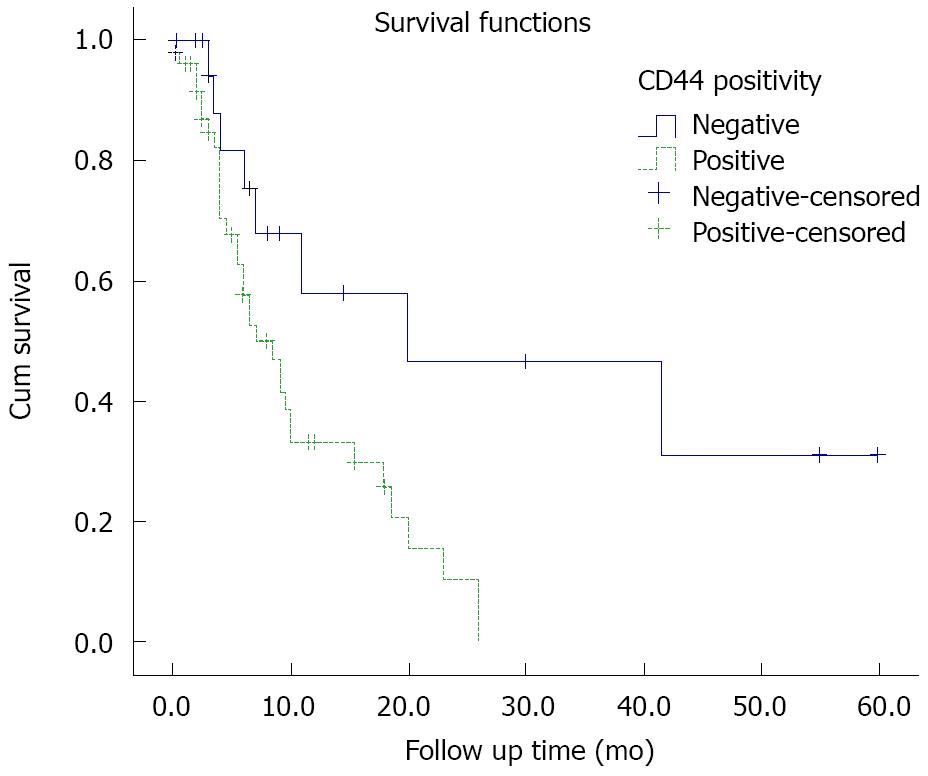
Follow-up data were gathered for 71 patients. The median follow-up time was 6 mo. The overall survival was 16 mo. Regarding the results of survival analysis, there was no correlation between sex, age, location, type and the grade of tumor with prognosis. Poor outcome was seen among the patients with higher stage tumors (stages III and IV, P = 0.034) compared to those with lower stages. In addition, the patients with CD44-positive tumors showed a poor prognosis (Log Rank, P = 0.008, Figure 2). Among patients with lower stage tumors, CD44 expression affected the prognosis regardless of tumor stage (P = 0.040). When analyzing the correlation of CD44 positivity, and intestinal/diffuse-type tumors separately, the results showed that CD44 only affected survival time in intestinal-type (P = 0.001), but not the diffuse-type tumors (P = 0.7).
The present study examined the expression of CD44 as an adhesion molecule in primary tumors by utilizing the IHC technique in 100 patients with gastric adenocarcinoma who had undergone surgery as the first step of management. The results showed that the expression rate of CD44 in tumoral tissue reached 65% in contrast to no expression of this molecule in the adjacent normal mucosa. CD44 was more commonly expressed in the intestinal-type tumor than in diffuse-type tumor, indicating that CD44 expression was related to the histological subtype of tumor, which is in agreement with previous studies[15,16]. In addition, a correlation was found between CD44 expression and tumor grade. But, no correlations between CD44 and other clinicopathological parameters were observed. Survival analysis showed that the grade and the histological type of gastric adenocarcinoma did not significantly affect survival. On the other hand, patients with higher stage adenocarcinoma showed a poor prognosis. CD44 was shown to affect survival, even more significantly than, and independently from the stage of the tumor. Moreover, CD44 positivity appeared to provide a poor prognosis only in patients with the intestinal-type gastric adenocarcinoma.
Since 1993 when Hieder et al[17] reported the expression of CD44 variants in gastric cancer, this adhesive molecule was recognized as a molecular marker related to the clinicopathological aspects of gastric cancer. CD44 is a member of a widely expressed family of adhesion receptors. This molecule was first described as a lymphocyte-homing receptor; however, it is expressed on a wide variety of cell types including mature T and B-cells[9,18-20]. Metastasizing tumor cells and recirculating (activated) lymphocytes share several properties, including motility and invasive behavior, an analogy which prompted the hypothesis that malignant cells might use molecules like CD44 for metastasizing[9]. CD44 gene is located on chromosome 11p12-13, having at least 20 exons, of which ten are expressed in hematopoietic cells or the standard form of the gene. Other exons can be alternatively spliced to make up a wide variety of CD44 splice variants which have been found in various types of human malignancies, and have been considered markers in tumor progression and metastasis[15].
It has been supposed that the evaluation of the CD44 isoforms expression by the IHC method in cases of non-Hodgkin lymphoma, colon and renal cell carcinomas, as well as neuroblastomas may be a useful diagnostic parameter indicating invasive processes[21]. In 1994, Yokozaki et al[22] indicated that the detection of CD44 transcription variants can serve as a powerful tool for the diagnosis of gastric cancer. Many studies have indicated that the generation of CD44 splice variants, like V5 and V6, might be linked closely to gastric carcinoma tumorigenesis and differentiation, suggesting that these isoforms can be used as an indicator of tumor progression in the biopsies of patients with gastric carcinoma[23-28]. Moreover, it has been shown that patients with an over-expression of CD44 have a higher lymph node metastatic rate and invasion[27].
It is possible that different results among studies reflect the use of diverse antibodies having subtle variations in specificity. The problem is complicated more by the existence of numerous CD44 isoforms, which may have remarkable homology in their antigenic repertoire, thus increasing the possibility of cross-reactivity between the antibodies. Another reason for such discrepancies is probably the comparison of results having different techniques[9].
The correlation between clinicopathological parameters and CD44 expression in the tumor is known to be different between intestinal and diffuse types of gastric carcinoma[29,30]. A study showed that the intestinal-type was more frequently CD44s- and CD44v6-positive than the diffuse-type tumor[15], although the reactivity to these two antibodies did not correlate with histopathological and clinical prognostic factors in intestinal-type carcinoma[31]. More recently, Yamaguchi et al[16] found that the expression of CD44v6 protein was significantly higher in differentiated adenocarcinoma than in diffuse-type carcinoma. On the other hand, Saito et al[29] observed that CD44v6 appeared to play an important role in the invasion and metastasis of diffuse-type gastric carcinoma, but not in that of intestinal-type gastric carcinoma, and also demonstrated a significant correlation between CD44v6 expression and poor prognosis of diffuse-type gastric carcinoma.
The results of this study are consistent with previous findings, demonstrating that CD44 is mostly expressed in the intestinal-type gastric cancer, and its expression is associated with poor prognosis. Thus, it can be concluded that the expression of CD44 is related to the phenotype of gastric malignancy, and may serve as a useful indicator of tumor metastasis, and may have a potential significance in diagnosing gastric cancer.
The mortality associated with gastric carcinoma is almost entirely caused by a subsequent metastatic disease. In fact, the prognostic assessment of gastric carcinoma still relies mainly on TNM staging, but the wide individual variability in prognosis is observed even in the same stages. The accurate prediction of the metastatic potential of the primary tumor, and hence the probable existence of undetected metastases, would be a critical factor in the management of patients with gastric carcinoma[14]. Our results emphasize that expression of CD44 is related to the prognosis of intestinal-type gastric cancer.
In conclusion, this study demonstrated that detection of CD44 protein in routinely fixed gastric carcinoma tissue by the IHC method can be used, along with other established parameters, to assess prognostic outcome, and particularly, to identify patients with a poor short-term prognosis. Furthermore, this suggests that, in the future, assessment of CD44 expression may guide the clinician in delineating a subset of patients with biologically unfavorable tumors who may profit from more intense post-operative adjuvant therapy.
Gastric cancer is the 2nd most common cause of cancer-related death in the world. The mortality associated with gastric carcinoma is almost entirely caused by a subsequent metastatic disease. The accurate prediction of the metastatic potential of the primary tumor would be a critical factor in the management of patients with gastric carcinoma. Metastasis is characterized by a loss of adhesion that allows cancer cells to invade and leave the site of origin, subsequently adhering to other sites such as lymph nodes, liver, or peritoneum. Cluster of differentiation 44 (CD44), as an important glycoprotein involved in cellular adhesion, is considered a determinant of metastatic and invasive potential in different malignancies. There are different and even contradictory reports considering the role of this adhesive molecule in gastric carcinogenesis and metastasis.
While there was no expression in surrounding normal tissue, CD44 expression rate in gastric adenocarcinoma was up to 65%, considering that the intestinal-type tumor expressed this marker, more common than diffuse-type tumor. Beside the prognostic effect of stage, CD44 was shown to affect survival, even more significantly than and independently from the stage of the tumor and this was more pronounced in intestinal-type.
For the first time in Iranian patients, this study demonstrated that detection of the CD44 protein in routinely fixed gastric carcinoma tissue by the IHC method can be used, along with other established parameters, to assess prognostic outcome, and particularly, to identify patients with a poor short-term prognosis.
In the future, assessment of CD44 expression may guide the clinician in delineating a subset of patients with biologically unfavorable tumors who may benefit from more intense postoperative adjuvant therapy.
The results showed that the over-expression of cell adhesion molecule CD44 is correlated with a poor prognosis in patients with the intestinal-type gastric adenocarcinoma. CD44 can, therefore, be utilized as a prognostic marker for this group of patients.
Peer reviewer: Robin G Lorenz, Associate Professor, Department of Pathology, University of Alabama at Birmingham, 845 19th Street South BBRB 730, Birmingham, AL 35294-2170, United States
S- Editor Zhong XY L- Editor Kumar M E- Editor Ma WH
| 1. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. |
| 2. | Abbaszadegan MR, Moaven O, Sima HR, Ghafarzadegan K, A’rabi A, Forghani MN, Raziee HR, Mashhadinejad A, Jafarzadeh M, Esmaili-Shandiz E. p16 promoter hypermethylation: a useful serum marker for early detection of gastric cancer. World J Gastroenterol. 2008;14:2055-2060. |
| 3. | Taghavi N, Nasrollahzadeh D, Merat S, Yazdanbod A, Hormazdi M, Sotoudeh M, Semnani S, Eslami F, Marjani HA, Fahimi S. Epidemiology of upper gastrointestinal cancers in Iran: a sub site analysis of 761 cases. World J Gastroenterol. 2007;13:5367-5370. |
| 4. | Yaghoobi M, Rakhshani N, Sadr F, Bijarchi R, Joshaghani Y, Mohammadkhani A, Attari A, Akbari MR, Hormazdi M, Malekzadeh R. Hereditary risk factors for the development of gastric cancer in younger patients. BMC Gastroenterol. 2004;4:28. |
| 5. | Panani AD. Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett. 2008;266:99-115. |
| 6. | Kountouras J, Zavos C, Chatzopoulos D, Katsinelos P. New aspects of Helicobacter pylori infection involvement in gastric oncogenesis. J Surg Res. 2008;146:149-158. |
| 7. | Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192-198. |
| 8. | Jothy S. CD44 and its partners in metastasis. Clin Exp Metastasis. 2003;20:195-201. |
| 9. | Zavrides HN, Zizi-Sermpetzoglou A, Panousopoulos D, Athanasas G, Elemenoglou I, Peros G. Prognostic evaluation of CD44 expression in correlation with bcl-2 and p53 in colorectal cancer. Folia Histochem Cytobiol. 2005;43:31-36. |
| 10. | Wang DR, Chen GY, Liu XL, Miao Y, Xia JG, Zhu LH, Tang D. CD44v6 in peripheral blood and bone marrow of patients with gastric cancer as micro-metastasis. World J Gastroenterol. 2006;12:36-42. |
| 11. | Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191-200. |
| 12. | Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893-904. |
| 13. | Setala L, Lipponen P, Tammi R, Tammi M, Eskelinen M, Alhava E, Kosma VM. Expression of CD44 and its variant isoform v3 has no prognostic value in gastric cancer. Histopathology. 2001;38:13-20. |
| 14. | Yoo CH, Noh SH, Kim H, Lee HY, Min JS. Prognostic significance of CD44 and nm23 expression in patients with stage II and stage IIIA gastric carcinoma. J Surg Oncol. 1999;71:22-28. |
| 15. | Dammrich J, Vollmers HP, Heider KH, Muller-Hermelink HK. Importance of different CD44v6 expression in human gastric intestinal and diffuse type cancers for metastatic lymphogenic spreading. J Mol Med. 1995;73:395-401. |
| 16. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. |
| 17. | Heider KH, Dammrich J, Skroch-Angel P, Muller-Hermelink HK, Vollmers HP, Herrlich P, Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993;53:4197-4203. |
| 18. | Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213-221. |
| 19. | Yoo CH, Noh SH. The Serum Assay of Soluble CD44 Standard, CD44 Variant 5, and CD44 Variant 6 in Patients with Gastric Cancer. Cancer Res Treat. 2003;35:3-8. |
| 20. | Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211-231. |
| 21. | Gunthert U, Stauder R, Mayer B, Terpe HJ, Finke L, Friedrichs K. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv. 1995;24:19-42. |
| 22. | Yokozaki H, Ito R, Nakayama H, Kuniyasu H, Taniyama K, Tahara E. Expression of CD44 abnormal transcripts in human gastric carcinomas. Cancer Lett. 1994;83:229-234. |
| 24. | Chen JQ, Zhan WH, He YL, Peng JS, Wang JP, Cai SR, Ma JP. Expression of heparanase gene, CD44v6, MMP-7 and nm23 protein and their relationship with the invasion and metastasis of gastric carcinomas. World J Gastroenterol. 2004;10:776-782. |
| 25. | Hsieh HF, Yu JC, Ho LI, Chiu SC, Harn HJ. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol Pathol. 1999;52:25-28. |
| 26. | Joo M, Lee HK, Kang YK. Expression of E-cadherin, beta-catenin, CD44s and CD44v6 in gastric adenocarcinoma: relationship with lymph node metastasis. Anticancer Res. 2003;23:1581-1588. |
| 27. | Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44s, CD44v6, and nm23 mRNA in human cancer. World J Gastroenterol. 2005;11:6601-6606. |
| 28. | Chen GY, Wang DR. The expression and clinical significance of CD44v in human gastric cancers. World J Gastroenterol. 2000;6:125-127. |
| 29. | Saito H, Tsujitani S, Katano K, Ikeguchi M, Maeta M, Kaibara N. Serum concentration of CD44 variant 6 and its relation to prognosis in patients with gastric carcinoma. Cancer. 1998;83:1094-1101. |
| 30. | Castella EM, Ariza A, Pellicer I, Fernandez-Vasalo A, Ojanguren I. Differential expression of CD44v6 in metastases of intestinal and diffuse types of gastric carcinoma. J Clin Pathol. 1998;51:134-137. |