GHRELIN AND THE REGULATION OF GASTRIC ACID SECRETION
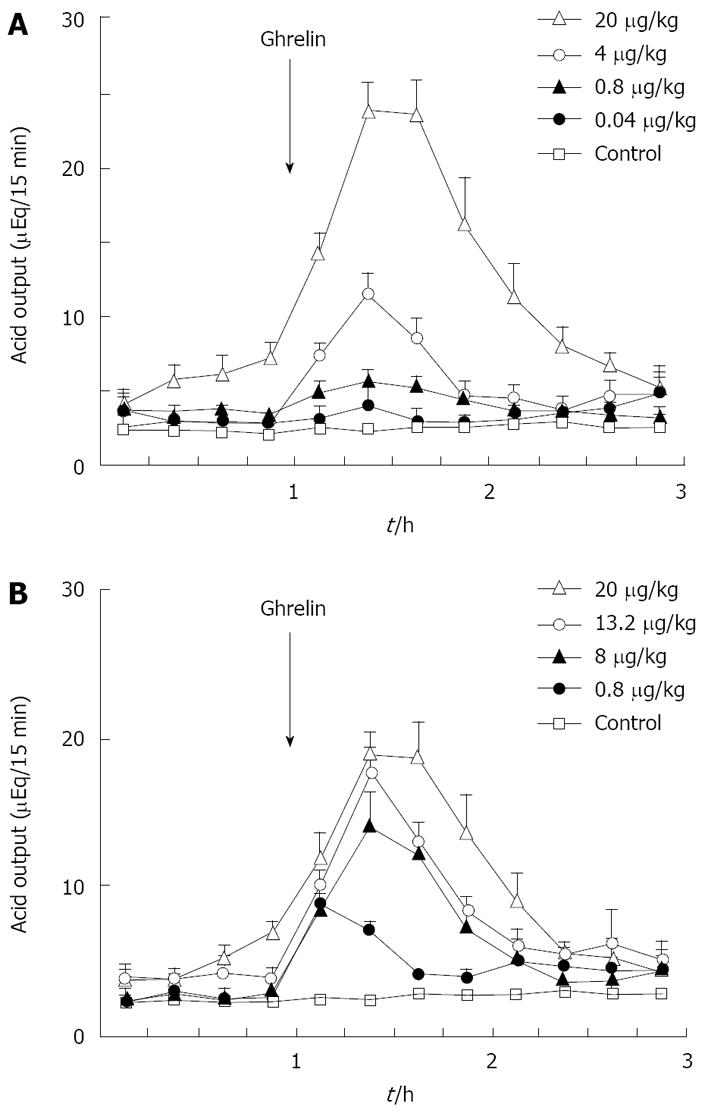
Figure 1 Effects of rat ghrelin (A) and gastrin-17 (B) on acid output in gastric lumen-perfused rats.
A: Time course of acid output by IV administration of ghrelin (0.04, 0.8, 4, 20 μg/kg) in gastric lumen perfused rats; B: Time course of acid output by IV administration of gastrin (0.8, 8, 13.2 and 20 μg/kg) in gastric lumen perfused rats. Saline was administered in control rats. Each value represents the mean ± SE of acid output at 15 min intervals. n = 4, 5, 6, 7. Reprinted with permission[30].
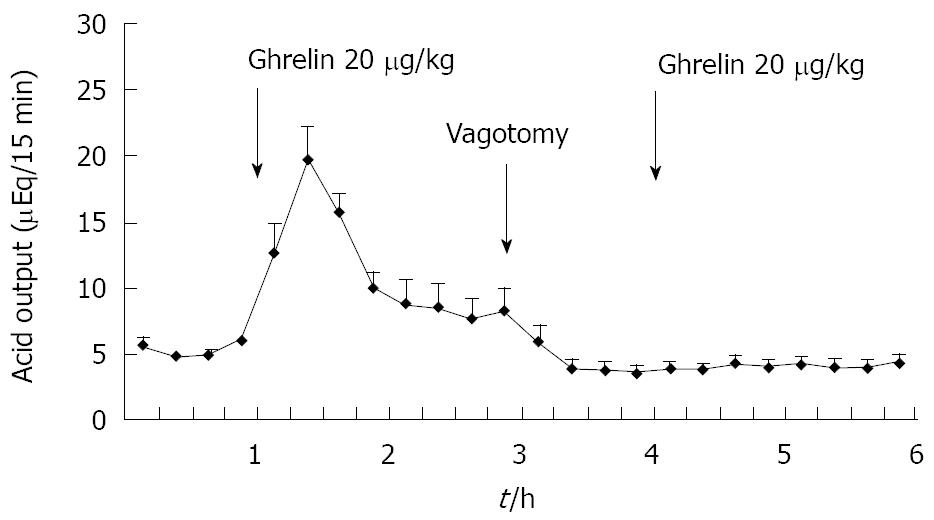
Figure 2 Effect of famotidine on ghrelin-stimulated acid output in gastric lumen-perfused rats.
Time course of acid output by the IV administration of famotidine (0.33 mg/kg) and ghrelin (20 μg/kg) in gastric lumen-perfused rats. Famotidine was administered intravenously 15 min before ghrelin injection. Each value represents the mean ± SE of acid output at 15 min intervals. n = 4. Reprinted with permission[30].
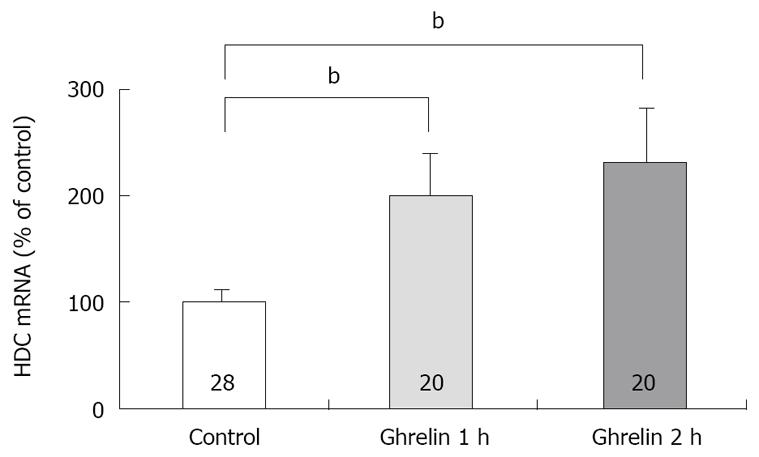
Figure 3 Effect of ghrelin on the concentration of HDC mRNA in rat gatsric mucosa.
Ghrelin (20 μg/kg) was administered intravenously. The concentration of HDC mRNA was measured by real time RT-PCR using LightCycler. Control: The concentration of HDC mRNA in gastric mucosa of rats with saline administration. 1 h; the concentration of HDC mRNA in gastric mucosa of rats with ghrelin administration, the stomachs were excised 1 h after the administration. 2 h; the concentration of HDC mRNA in gastric mucosa of rats with ghrelin administration, the stomachs were excised 2 h after the administration. Each value represents the mean ± SE of HDC mRNA demonstrated as % of control. n = 28, 20, 20. bP < 0.01. Reprinted with permission[30].
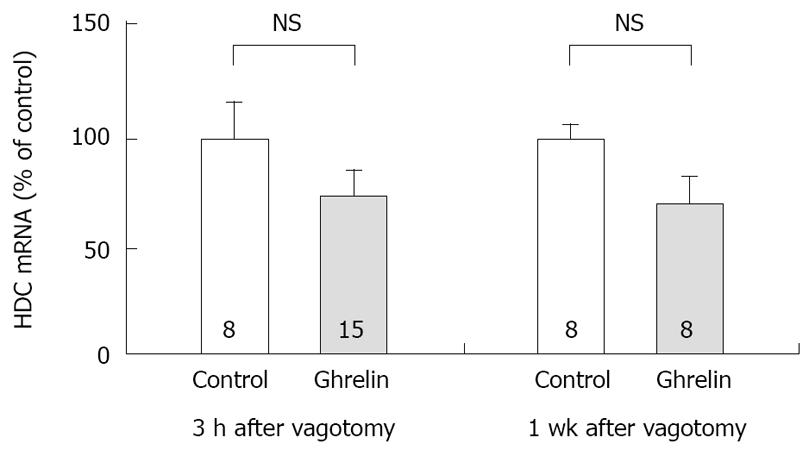
Figure 4 Effect of vagotomy on the concentration of HDC mRNA in rat gastric mucosa stimulated by ghrelin administration.
Subdiaphragmatic vagotomy was performed in rats 3 h or 7 d before the experiments. Ghrelin (20 µg/kg) was administered IV. The concentration of HDC mRNA was measured by real time RT-PCR using Light Cycler. Control: The concentration of HDC mRNA in gastric mucosa of rats with saline administration. Ghrelin: The concentration of HDC mRNA in gastric mucosa of rats with ghrelin administration, the stomachs were excised 2 h after the administration. 3 h after vagotomy, the experiments were performed 3 h after vagotomy. 1 wk after vagotomy, the experiments were performed 7 d after vagotomy. Each value represents the mean ± SE of HDC mRNA demonstrated as % of control. n = 8 or 15. NS: Not significant. Reprinted with permission[30].
Ghrelin, a novel growth-hormone-releasing peptide, was originally isolated from rat and human stomachs[1] and was demonstrated to localize in the endocrine cells of the stomach and hypothalamus[1,2]. Ghrelin has been known as a multifunctional hormone. Ghrelin increases secretion of growth hormone (GH), food intake, and body weight gain when administered peripherally or centrally[1,3-9]. Ghrelin has also positive cardiovascular effects. In humans, infusion of ghrelin decreases systemic vascular resistance and increases cardiac output in patients with heart failure. On the functions of stomach, ghrelin was also known to stimulate gastric motility and the secretion of gastric acid when administered peripherally or centrally[10,11]. As ghrelin has such multiple actions on many organs, physiological roles of ghrelin in vivo might be important even if many of them are not elucidated.
The mechanism for the action of ghrelin on feeding, growth hormone secretion and the secretion of gastric acid was studied and demonstrated that the vagal nerve was involved in the action of ghrelin[10-12]. When ghrelin was administered peripherally, ghrelin induced c-fos expression in the neurons of the arcuate nucleus of rats[12]. However, ghrelin did not induce c-fos expression in the neuron in both capsaicin-treated rats and vagotomized rats[12]. The effects of ghrelin on feeding were also abolished with capsaisin treatment and vagotomy[12]. These results suggest that gastric vagal afferent is the major pathway conveying ghrelin’s signals for starvation and GH secretion to the brain. On the action of ghrelin on the secretion of gastric acid, vagal nerve was indicated to be involved in the action of ghrelin[10]. Masuda and co-workers indicated that the action of ghrelin on the secretion of gastric acid was abolished by the pretreatment with atropine or bilateral cervical vagotomy[10]. Date and co-workers demonstrated that intracerebroventricular (ICV) administration of ghrelin induced the increase in the secretion of gastric acid and vagotomy and the pretreatment with atropine abolished the action of ghrelin[11]. They also demonstrated that ICV administration of ghrelin induced c-fos expression in the neurons of the nucleus of the solitary tract (NTS) and the dorsal motor nucleus of the vagus nerve (DMX)[11]. Taken together, these results suggest that vagal nerve plays important roles in the action of ghrelin on the secretion of gastric acid.
GHRELIN AND HISTAMINE RELEASE
As dictated above, ghrelin stimulates the secretion of gastric acid[10,11]. The mechanism for the action of ghrelin on acid secretion was also demonstrated that the vagal nerve was involved in the action of ghrelin[10,11]. However, the details of the mechanism after the activation of vagal nerve by ghrelin administration remains to be elucidated.
In the mechanism for acid secretion, vagal nerve has been known to play an important role, especially in the regulation by central nervous system[13] and in the stimulation by the distenstion of stomach[14]. Vagal nerve has stimulatory and inhibitory actions on acid secretion[15,16] and it contains and releases several neurotransmitters such as acetylcholine[17], gastrin-releaseing peptide (GRP)[18], substance P[19]. Calcitonin gene-related peptide[19] and pituitary adenylate cyclase activating peptide (PACAP)[20]. Among these, GRP has a stimulatory action on gastrin release from G cells[21]. Gastrin released by GRP stimulation is the most important physiological secretagogue and it has a primary role in postprandial acid secretion[22]. Gastrin stimulates acid secretion via enterochromaffin-like cells (ECL cell)[23]. Another transmitter, PACA, was also found to increase histamine release from ECL cell[24]. PACAP-immunoreactive nerve fibers are abundant in the gastric mucosa of both rat and humans[25] and gastric ECL cells possesses PACAP receptors(PAC1)[26]. Gastrin is now known as major stimulant of histamine release from gastric ECL cells[27]. Histamine released from ECL cells acts on parietal cells through H2 receptor[28]. It was indicated that the activation of vagal nerve induced an increase in histamine release from gastric mucosa[29]. Accordingly it is plausible that histamine release may be involved in the increased acid secretion induced by administration of ghrelin.
In a previous report, Masuda et al[10] demonstrated that vagal nerve is involved in the action of ghrelin on acid secretion. However, they concluded that histamine was not involved in the action of ghrelin on acid secretion, as famotidine did not inhibited ghrelin-induced acid secretion[10]. Tthe mechanism that was demonstrated by Masuda seemed to require further investigation, because the vagal stimulation[29], as well as transmitter PACAP, is known to increase release of histamine from ECL cells[24]. Therefore, if ghrelin administration stimulates the vagal nerve, an increase in histamine release would consequently occur.
Previously we attempted to clarify whether histamine is involved in the action of ghrelin[30]. In our study, ghrelin (0.8-20 μg/kg) dose-dependently increased gastric acid secretion and the action of ghrelin (20 μg/kg, 6 × 10-9 mol/kg) on acid secretion was almost as efficient as that of gastrin (20 μg/kg, 9.2 × 10-9 mol/kg) (Figure 1) [30]. The study demonstrated that vagotomy abolished the increase in acid secretion by ghrelin administration (Figure 2)[30] and also that famotidine completely inhibited the stimulatory action of ghrelin [30]. Furthermore, administration of ghrelin significantly increased HDC mRNA concentration of gastric mucosa (Figure 3) [30]. Vagotomy also abolished the increase in HDC mRNA by ghrelin administration (Figure 4) [30]. Furthermore, in the study, isolated vascularly-perfused stomachs that lacked vagal innervation were prepared in order to examine the effect of ghrelin on histamine release. Although the infusion of gastrin (2.1 μg/10 min) increased histamine release, ghrelin (5 μg/10 min) did not induce histamine release from isolated rat stomachs. Taken together, the results demonstrate the mechanism of action of ghrelin involves the vagal nerve as well as increases in release and synthesis of histamine, consequently induces the stimulation of parietal cells.
The reason that Masuda et al did not observe the inhibitory effect of famotidine on the action of ghrelin is unclear. However, there is a difference between the administration methods in the study by Matsuda et al and those used in our study. We administered ghrelin intravenously, while Matsuda et al administered ghrelin subcutaneously. The difference in the results may thus be due to the difference in the administration methods.
GHRELIN AND GASTRIN IN THE MECHANISM FOR THE REGULATION OF GASTRIC ACID SECRETION
Gastrin is known to be released into the circulation in response to food[31], and to play an important roles in increasing postprandial acid secretion[22], and to exert activity on ECL cells[23]. It was shown that vagotomy did not affect the maximal response of acid secretion to gastrin administration thus indicating that the action of gastrin was not primarily dependent on vagal nerve[32]. On the other hand, ghrelin is released during fasting period, and food intake suppresses its release[33]. As vagotomy completely abolished ghrelin-induced acid secretion and HDC mRNA production, ghrelin is thought to induce histamine release via vagal nerve activation, consequently resulting in increased acid secretion by parietal cells. Accordingly the mechanism for the secretion of ghrelin appears to differ from that of gastrin.
Recently, however, synergistic action of ghrelin and ghrelin on gastric acid secretion was reported from two groups[34,35]. Fukumoto et al have shown that IV administration of gastrin induced transient increases of ghrelin levels within 10 minutes and that simultaneous administration of both gastrin and ghrelin resulted in a synergistic increase of gastric acid secretion in rat[35]. They supposed that gastrin may directly stimulate ghrelin release and both hormones may increase gastric acid secretion synergistically[35]. We also presented the data that shows synergistic effects of gastrin and ghrelin on gastric acid secretion and histamine production by gastric mucosa which involves the vagal nerve[34]. Circulating ghrelin decreases soon after the initiation of feeding, gastrin oppositely increase with food intake. Generally simultaneous increases of gastrin and ghrelin do not occur. Therefore physiological role of this synergistic action of these hormones remain to be clarified. We are supposing this synergistic action may occurs in subjects with Hp infection that induces hypergastrinemia in fasting when ghrelin level rises.
CENTRAL REGULATION OF GASTRIC ACID SECRETION BY GHRELIN
The peripheral vagal nerve and the nuclei of central nerves are thought to be involved in the mechanism of action of ghrelin[6,12]. Ghrelin administered IV induced Fos expression only in the neurons of the arcuate nucleus of the hypothalamus in rats[12]. On the other hand, ICV injection induced Fos expression in the neurons of NTS and DMX of the medulla oblongata and other nuclei such as several hypothalamic nuclei, the dentate gyrus, the hippocampus, and the cerebral cortex[6,11]. The hypothalamus is known to be the center for hunger and satiety[36]. In particular, the arcuate nucleus of the hypothalamus is activated by ghrelin administration and this region is known to have important role in controlling food intake relating to the action of leptin[37]. Date et al[12] indicated that IV injection of ghrelin induces Fos expression in neuropeptide Y (NPY)-producing and growth hormone-releasing hormone (GHRH)-producing neurons in the arcuate neucleus. It is known that injection of NPY into cerebral ventricles or directly into the hypothalamus of rats potently stimulates food intake[38]. Therefore, the activation of NPY neuron in the arcuate nucleus of the hypothalamus by ghrelin is thought to relate to the stimulatory effect of ghrelin on food intake. However, it is still unclear whether activation of the arcuate nucleus induces increase in the secretion of gastric acid. It has been shown that ghrelin receptors are synthesized in vagal afferent neurons and transported to the afferent terminals[12]. Date et al[12] suggested that the gastric vagal afferent nerve, which is capsaicin sensitive, is the major pathway conveying ghrelin signals for starvation and growth hormone secretion to the brain. It is possible that the same pathway mediates the mechanism for the action on the secretion of gastric acid when ghrelin is administered IV. On the other hand, as described above, DMX, NTS and several nuclei of the brain are apparently activated by ICV administration of ghrelin[6,11]. As DMX has been demonstrated to relate to the secretion of gastric acid, the activation of DMX may be related to the central nervous system mechanism for the action of ghrelin on acid secretion. Furthermore, vagotomy also abolished the stimulatory effect of ICV administration of ghrelin on acid secretion, thus the vagal efferent may also be involved in the action of cerebral ghrelin[11].
The neuronal pathway mediating the peripheral ghrelin appears to be different from that mediating central ghrelin. Although the vagal afferent nerve was demonstrated to be involved in the action of peripheral ghrelin[10,12], the role of vagal efferent nerve remained to be elucidated. However, as the action of cerebral ghrelin is thought to involve vagal nerve, possibly vagal efferent nerve, and the results of our previous study demonstrated that the actions of ghrelin on acid secretion and HDC mRNA production require the vagal nerve to be intact, it is possible that vagal efferent nerve is also involved in the action of peripheral ghrelin. A likely hypothesis based on the results of our study is that peripheral ghrelin stimulation of gastric acid secretion initiates the activation of central regulatory system that induces the activation of vagal nerve, resulting in possibly release of neurotransmitters and ECL cells stimulation.
Recently, as to the action of ghrelin, involvement of NO synthesis has been indicated[40]. Bilgin et al reported that ghrelin stimulates the secretion of gastric acid through NO as a mediator, because acid secretion induced by ghrelin administration was inhibited by applying L-NAME in rats. Previously the gastroprotective effect of ghrelin was reported and this can be attenuated by pretreatment of L-NAME suggesting involvement of NO pathways in the effect of ghrelin[40]. As NO increases mucosal blood flow, the effect of ghrelin on the secretion of gastric acid may partially dependent of an increase of mucosal blood flow.
THE PHYSIOLOGICAL ROLE OF GHRELIN IN THE REGULATION OF ACID SECRETION
The physiological role of ghrelin in the regulation of acid secretion still remains unclear. Comparing the secretion of ghrelin with that of gastrin, the secretion of gastrin is induced by food intake[26], while the secretion of ghrelin is increased during fasting period[33]. As described above, there are many differences between gastrin and ghrelin in the actions, their roles may be different. Gastrin is known to play important roles in the postprandial secretion of gastric acid, while ghrelin may be related to acid secretion during fasting period or at night. To elucidate the physiological role of ghrelin in acid secretion, further studies are required.
S- Editor Xiao LL E- Editor Ma WH