Published online Oct 28, 2008. doi: 10.3748/wjg.14.6261
Revised: October 16, 2008
Accepted: October 23, 2008
Published online: October 28, 2008
A case of a successfully treated solitary fibrous tumor (SFT) of the liver is reported. An 82-year-old female presented with left upper abdominal discomfort, a firm mass on palpation, and imaging studies revealed a large tumor, 15 cm in diameter, arising from the left lobe of the liver. A formal left hepatectomy was performed. Microscopic evaluation showed spindle and fibroblast-like cells within the collagenous stroma. Immunohistochemistry disclosed diffuse CD34 and positive vimentin, supporting the diagnosis of a benign SFT. The patient remained well 21 months after surgery. SFT of the liver is a very rare neoplasm of mesenchymal origin. In most cases it is a benign lesion, although some may have malignant histological features and recur locally or metastasize. With less than 30 reported cases in the literature, little can be said regarding its natural history or the benefits of adjuvant radiochemotherapy. Complete surgical resection remains the cornerstone of its treatment.
- Citation: Korkolis DP, Apostolaki K, Aggeli C, Plataniotis G, Gontikakis E, Volanaki D, Sebastiadou M, Dimitroulopoulos D, Xinopoulos D, Zografos GN, Vassilopoulos PP. Solitary fibrous tumor of the liver expressing CD34 and vimentin: A case report. World J Gastroenterol 2008; 14(40): 6261-6264
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6261.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6261
Solitary fibrous tumor (SFT) is a rare neoplasm of mesenchymal origin that occurs preferentially in the pleura, meninges, orbit, upper respiratory tract, thyroid and peritoneum. In extremely unusual cases, SFT may arise from the liver parenchyma of adult patients[1]. In the English literature, less than 30 cases of SFTs of the liver have been reported[2-5].
Clinical or radiological findings are not specific and cannot exclude malignancy. Preoperative cytology may be inconclusive or misleading. Immunohistologically, CD34, vimentin and desmin should be used as markers to precisely diagnose an SFT of the liver[3]. In most cases, there is low cellularity with minimal atypia or necrosis, making this a benign entity. Occasionally, a large size, high mitotic rate, cellular pleomorphism, atypia and central necrosis are interpreted as features suggestive of malignant behavior. As a result of its rarity, overall experience is insignificant. The outcome of an SFT of the liver is mostly related to resectability, although correlated with neither pathological grade nor tumor size[4]. Thus, complete surgical removal of the neoplasm is most commonly proposed. We describe a new case of SFT of the liver and review the literature.
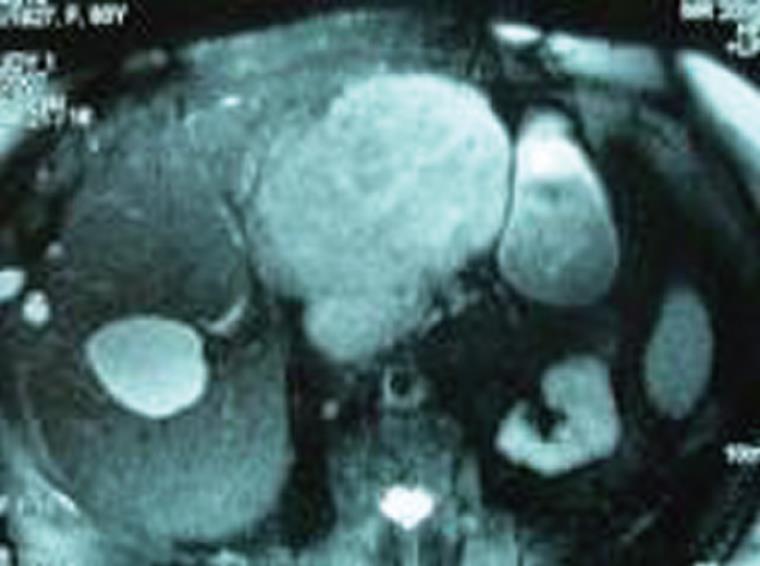
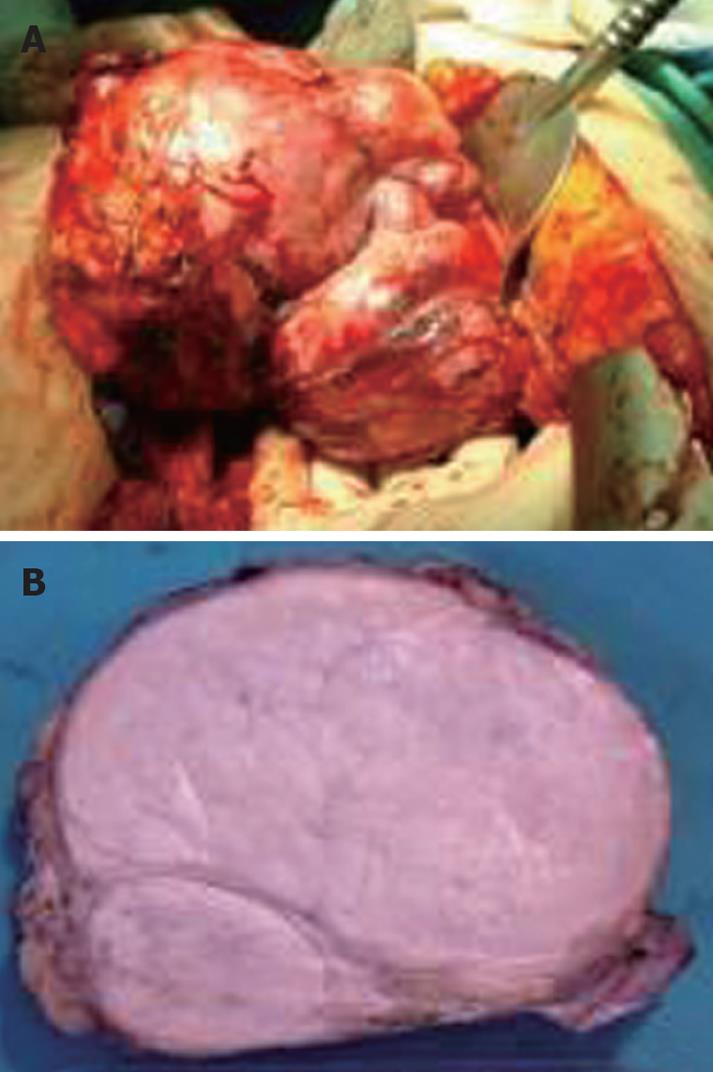
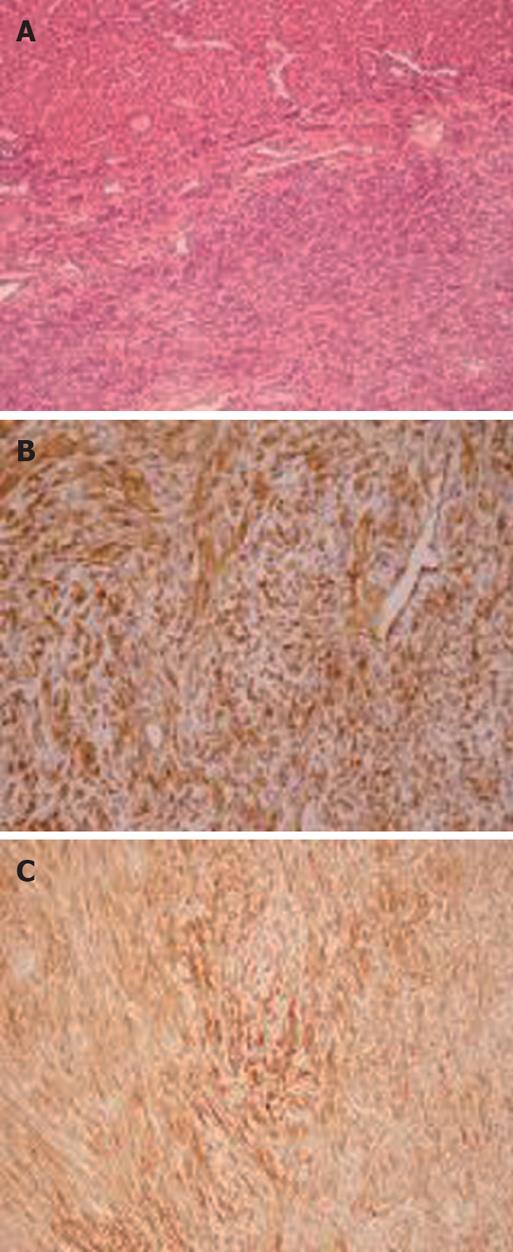
An 82-year-old female patient was referred to our institution on account of persistent abdominal discomfort and a palpable firm mass in the left upper quadrant. She had a past medical history of severe sleep-apnea syndrome and was recovering from a recent episode of mild gallstone pancreatitis. Laboratory tests, including liver biochemical profile and tumor markers were within normal range. Ultrasonography revealed an ovoid mass in the left lobe of the liver. Dynamic computed tomography (CT) and magnetic resonance imaging demonstrated a large, space-occupying lesion arising from the left lobe and compressing the stomach, pancreas and hepatoduodenal ligament. The lesion showed an early arterial enhancement and delayed venous washing out. The tumor developed in a polycystic liver parenchyma. It was well defined and measured about 15 cm in diameter. No direct invasion of the great vessels or adjacent intraperitoneal structures was seen (Figure 1). Preoperative fine needle aspiration biopsy under CT guidance, performed in another institution, was suggestive of a primary hepatocellular carcinoma. The patient underwent formal left hepatectomy and cholecystectomy. On gross examination, the resected specimen measured 18 cm × 15 cm × 8 cm and weighed 1275 g (Figure 2A). On the cut section, the tumor was firm, lobulated, well-demarcated, greyish-white, with a whorled and fasciculated surface and focal myxoid degeneration (Figure 2B). No cirrhosis or fibrosis was observed in the peripheral liver parenchyma. Resection margins were free of tumor. Pathological examination showed a highly cellular neoplasm composed of monomorphic spindle and fibroblast-like cells arranged in a storiform pattern. They were intermingled with dense bundles of collagen. In some areas, tumor cells were arranged around ectatic vessels in a hemangiopericytoma-like pattern (Figure 3A). No mitotic figures or nuclear polymorphism were noted. Immunohistochemical studies revealed a strong cytoplasmic positivity for CD34 (Figure 3B), vimentin (Figure 3C), desmin and Bcl-2. An absence of staining for HHF-35, S-100, CD117, muscle-specific actin and cytokeratin was observed. The percentage of ki-67 positive tumor cells was less than 5%. On the basis of the aforementioned results, pathological evaluation ascertained the presence of a benign SFT of the liver.
The patient recovered uneventfully after the procedure. She was discharged home on the 6th postoperative day and followed-up on an outpatient basis. Twenty-one months after surgery, the patient was stll doing well with no evidence of recurrence.
SFTs of the liver are unusual neoplasms with fewer than 30 previous cases reported in the world literature. There is a 2:1 female-to-male predominance with the ages of affected individuals ranging from 16 to 83 years. The mean age at diagnosis is 55 years and the average follow-up period reaches 27 months[1]. Most SFTs of the liver are usually found as giant lesions growing in either the right or the left lobe of a non-cirrhotic liver, causing non-specific symptoms of fullness and pressure, gastrointestinal obstruction, weight loss or hypoglycemia[3,5]. In addition, the development of a primary SFT on a background of polycystic liver disease, as in the present case, has never been reported before.
As a result of their extreme rarity, overall experience of SFTs is limited. In addition, the difficulty of interpretation of histological pictures and the huge volume at presentation still raise problems in terms of correct preoperative diagnosis and proper clinical management[6]. In the present case, the diagnosis of SFT of the liver was based on the association of characteristic histological and immunohistochemical features, i.e. high cellular proliferation of spindle cells arranged in a storiform pattern, together with the immunohistochemical staining profile of CD34 (+), vimentin (+), Bcl-2 (+) and cytokeratin (-), which is highly suggestive for SFTs. These characteristics differentiate it from other liver tumors, such as primary hepatocellular carcinoma (CD34-negative), leiomyoma (smooth-muscle actin-positive and CD34-negative), inflammatory pseudotumor (forms fibrous tissue made by collagen fibers with fibro and myofibroblast and plasma cells), fibrosarcoma (forms a “herring bone” pattern), and epithelioid hemangioendothelioma (factor VIII positivity)[7]. The pathological features described for the presented case (low mitotic rate, no nuclear atypia and cellular pleomorphism) are in keeping with those of a benign SFT of the liver. This was confirmed by the favorable course of the patient, who was alive and disease-free 21 mo after surgery.
Radical surgical removal of the tumor with clear margins of resection is the mainstay of treatment. Since most SFTs of the liver are usually found to represent large, well-circumscribed lesions, complete removal necessitates the performance of major hepatectomies (≥ 3 segments). Owing to the size of most SFTs of the liver and their tendency to displace surrounding structures, they are virtually altering the intra-abdominal anatomy in an unpredictable manner. Although an SFT is not a primarily malignant disease, it seems to be of utmost importance that tumor-free resection margins be achieved in order that locoregional recurrence is prevented[8,9]. In light of this, surgical treatment of a hepatic SFT poses a tremendous challenge to the liver surgeon.
Little can be written about the possible benefits of adjuvant radio- and/or chemotherapy in these patients, as reported data are scarce. As SFT of the liver is often a benign neoplasm, postoperative chemotherapy or radiotherapy should not be necessary and is reserved for when resection is incomplete or pathological examination reveals features of malignancy[3,10].
In conclusion, SFTs of the liver are extremely rare neoplasms in adult patients and their incidence is unknown. Precise diagnosis is solely based on the correct interpretation of unique pathological and immunohistochemical features. Aggressive surgical management remains the treatment of choice. The outcome of SFTs is mostly related to resectability, rather than on pathologic grade or tumor size. Since the current number of reported cases is very limited, all efforts should be made to ensure careful follow-up of all identified patients. Only then we can reliably comment on the behavior and definite prognosis of SFTs of the liver.
Peer reviewers: Barry Rosser, MD, Department of Transplantation, Mayo Clinic Jacksonville, Suite 1100, 4205 Belfort Road, Jacksonville, Florida 32216, United States; Michael Torbenson, MD, Associate Professor of Pathology, Room B314, 1503 E Jefferson (Bond Street Building), The Johns Hopkins University School of Medicine, Baltimore, MD 21231, United States
S- Editor Xiao LL L- Editor Wang XL E- Editor Lin YP
| 1. | Neeff H, Obermaier R, Technau-Ihling K, Werner M, Kurtz C, Imdahl A, Hopt UT. Solitary fibrous tumour of the liver: case report and review of the literature. Langenbecks Arch Surg. 2004;389:293-298. |
| 2. | Changku J, Shaohua S, Zhicheng Z, Shusen Z. Solitary fibrous tumor of the liver: retrospective study of reported cases. Cancer Invest. 2006;24:132-135. |
| 3. | Vennarecci G, Ettorre GM, Giovannelli L, Del Nonno F, Perracchio L, Visca P, Corazza V, Vidiri A, Visco G, Santoro E. Solitary fibrous tumor of the liver. J Hepatobiliary Pancreat Surg. 2005;12:341-344. |
| 4. | Ji Y, Fan J, Xu Y, Zhou J, Zeng HY, Tan YS. Solitary fibrous tumor of the liver. Hepatobiliary Pancreat Dis Int. 2006;5:151-153. |
| 5. | Guglielmi A, Frameglia M, Iuzzolino P, Martignoni G, De Manzoni G, Laterza E, Veraldi GF, Girlanda R. Solitary fibrous tumor of the liver with CD 34 positivity and hypoglycemia. J Hepatobiliary Pancreat Surg. 1998;5:212-216. |
| 6. | Saint-Marc O, Pozzo A, Causse X, Heitzmann A, Debillon G. [Solitary fibrous liver tumor: clinical, radiological and pathological characteristics]. Gastroenterol Clin Biol. 2002;26:171-173. |
| 7. | Barnoud R, Arvieux C, Pasquier D, Pasquier B, Letoublon C. Solitary fibrous tumour of the liver with CD34 expression. Histopathology. 1996;28:551-554. |
| 8. | Nath DS, Rutzick AD, Sielaff TD. Solitary fibrous tumor of the liver. AJR Am J Roentgenol. 2006;187:W187-W190. |
| 9. | Yilmaz S, Kirimlioglu V, Ertas E, Hilmioglu F, Yildirim B, Katz D, Mizrak B. Giant solitary fibrous tumor of the liver with metastasis to the skeletal system successfully treated with trisegmentectomy. Dig Dis Sci. 2000;45:168-174. |
| 10. | Kwak SY, Gwak GY, Yun WK, Kim HJ, Do IG, Joh JW, Park CK. [A case of solitary fibrous tumor of the liver]. Korean J Hepatol. 2007;13:560-564. |