Published online Oct 28, 2008. doi: 10.3748/wjg.14.6254
Revised: July 14, 2008
Accepted: July 21, 2008
Published online: October 28, 2008
AIM: To detect the expression of DPC4 in malignant and non-malignant specimens of human pancreas, and observe the inhibition of retroviral pLXSN containing DPC4 on pancreatic carcinoma cells in vitro.
METHODS: The expression of DPC4 was determined in 40 pancreatic adenocarcinoma and 36 non-malignant pancreatic specimens by reverse-transcriptase polymerase chain reaction (RT-PCR) and immunohisto-chemistry. Furthermore, we constructed retroviral vectors containing DPC4, which then infected the pancreatic carcinoma cell line BxPC-3. Cell growth in vitro after being infected was observed, and the vascular endothelial growth factor (VEGF) mRNA level in the daughter cells was determined by semi-quantitative PCR assay.
RESULTS: The RT-PCR assay showed a positive rate of DPC4 mRNA in 100% (36/36) of normal specimens, compared to 40% (16/40) in adenocarcinoma specimens. The regional and intense positive cases of DPC4 expression in adenocarcinoma detected by immunohistochemistry were 10 and four, whereas it was all positive expression in normal tissues. There was a significant difference of DPC4 expression between them. The stable expression of DPC4 in the pancreatic carcinoma cells BxPC-3 could be resumed by retroviral vector pLXSN transfection, and could inhibit cell growth in vitro. Rather, DPC4 could decrease VEGF mRNA transcription levels.
CONCLUSION: The deletion of DPC4 expression in pancreatic carcinoma suggests that loss of DPC4 may be involved in the development of pancreatic carcinoma. The retroviral vector pLXSN containing DPC4 can inhibit the proliferation of pancreatic carcinoma cells, and down-regulate the level of VEGF.
-
Citation: Shen W, Tao GQ, Li DC, Zhu XG, Bai X, Cai B. Inhibition of pancreatic carcinoma cell growth
in vitro by DPC4 gene transfection. World J Gastroenterol 2008; 14(40): 6254-6260 - URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6254.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6254
| BxPC-3/DPC4 | Bxpc-3/pLXSN | Bxpc-3/- | Ratio | |
| VEGF | 0.1887 ± 1.2399 | 0.3875 ± 2.0478 | 0.3910 ± 1.0714 | 2.0535 |
| 1P | 0.035 | 0.021 |
Chromosome 18q is lost in a high proportion of colorectal and pancreatic cancers. Three candidate tumor suppressor genes, DCC (deleted in colorectal carcinoma), DPC4 (deleted in pancreatic carcinoma, locus 4) and Smad2 have been identified in this chromosome region. The tumor suppressor DPC4, which was identified by Scott Kern in 1996[1], is frequently lost in many tumor cells, especially in pancreatic cells. DPC4, also named as Smad4, belongs to the evolutionarily conserved family of Smad proteins that are crucial intracellular mediators of signals from transforming growth factor-β (TGF-β)[2]. TGF-β regulates a wide variety of biological activities. Smad proteins can transduce the TGF-β signal at the cell surface into gene regulation in the nucleus.
Here, we detected the expression of DPC4 in 40 pancreatic adenocarcinoma and 36 non-malignant pancreatic specimens by RT-PCR and immunohistochemistry; then, we reintroduced the DPC4 gene in the pancreatic carcinoma cell line BxPC-3 (null for DPC4[3]), by transferring the retroviral vector pLXSN containing the DPC4 gene, in order to study inhibition of DPC4 gene expression in the pancreatic carcinoma cells in vitro.
Forty malignant pancreatic carcinoma and 36 corresponding non-cancerous tissues were obtained from the First Affiliated Hospital of Suzhou University and the Wuxi’s People Hospital from 2003 to 2006. The clinical and pathological data from this patient population were readily available from pathology reports and a regularly updated clinical database. There were 28 males and 12 females with pancreatic carcinomas, and the average age of the patients was 55.18 ± 11.29 years old (mean ± SD). Tumor fragments were obtained in sterile conditions from different areas of the specimen and immediately placed in supplemented RPMI-1640 medium.
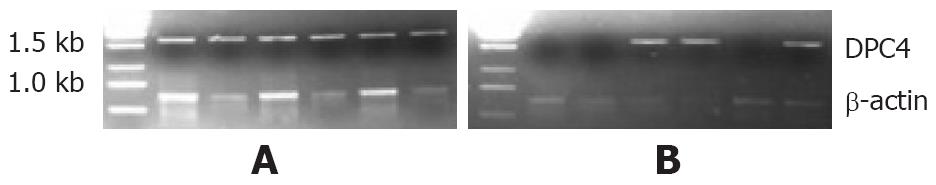
Total RNA was extracted from tissues with a single-step method. Randomly primed cDNAs were reverse-transcribed from 4 μg total RNA, which was extracted from about 1 g fresh specimen, using a cDNA synthesis kit in a 20-μL mixture. The 2-μL mixture was increased to 100 μL by adding 10 mmol/L Tris-HCI (pH 8.3), 50 mmol/L KCl2, 1.5 nmol/L MgCl2, 200 μL of each deoxynucleotide triphosphate, 6 U Taq polymerase, and 50 pmol of each of the specific oligonucleotide primers for DPC4. PCR amplification was performed in a DNA thermal cycler and consisted of 30 or 40 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C, and extension for 90 s 68°C. Final extension proceeded for 1 min at 68°C, Internal control for RNA quality was obtained with β-actin, which was amplified at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min for 30 cycles. PCR amplification was performed by using primers 5'-CGGAATTCATGGACAATATGTCTATTACG-3' and 5'-GCGGATCCTCAGTCTAAAGGTTGTGG-3' for the DPC4 cDNA fragment. The product was about 1.6 kb. The PCR primers for β-actin were 5'-ACACTGTGCCCATCTACGAGG-3' and 5'- AGGGGCCGGACTCGTCATACT-3'. The product was 621 bp. All the products were run on a 1% agarose gel and visualized by ethidium bromide staining.
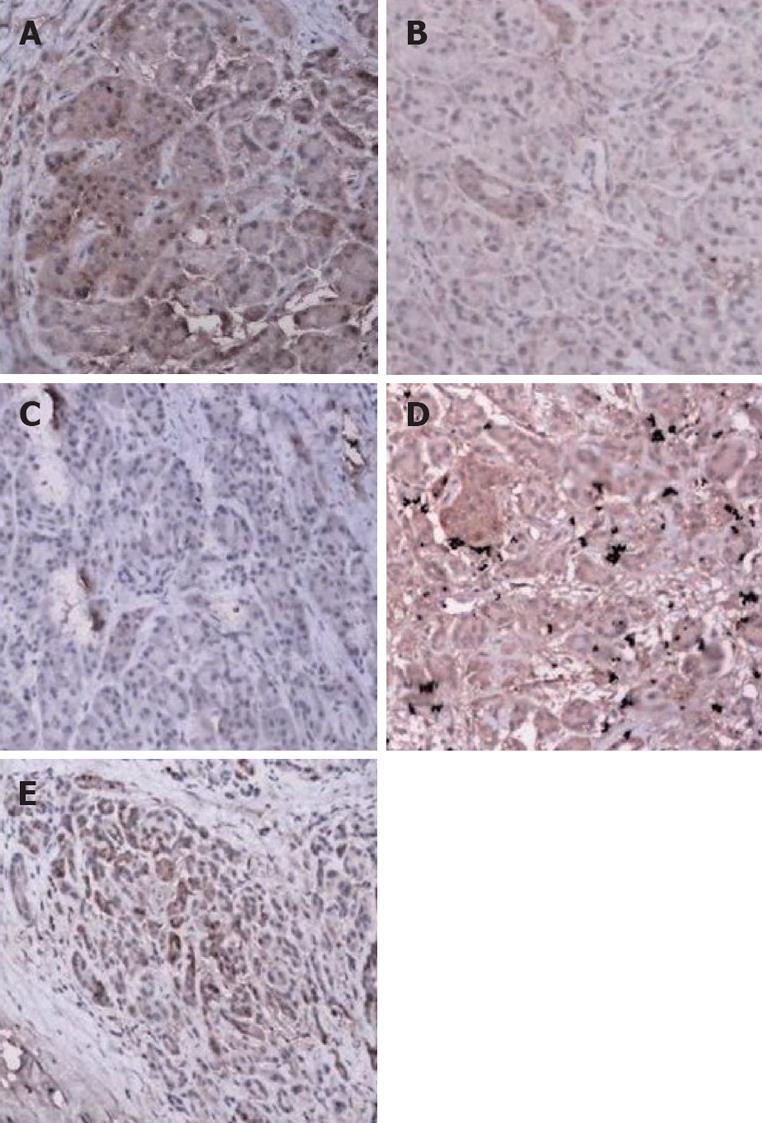
Tissues were routinely fixed in neutral formalin and embedded in paraffin. After being deparaffinized, the slides were placed in a solution of 3% hydrogen peroxide for 5-10 min to block the activity of endogenous peroxidase. After being washed with distilled water and then dipped in PBS for 5 min, the slides were closed in normal sheep blood serum and then heated to room temperature for 10 min. Non-specific binding was blocked with a protein solution for 10 min, and then each slide was labeled with a 1:100 dilution of monoclonal antibody to DPC4 (murine anti-human; LAB Vision). Anti-DPC4 antibody was detected by adding secondary antibodies (rabbit anti-murine; Maxim Biotech, Fuzhou). After being incubated at 37°C for 1 h, the slides were washed by PBS. The sections were counterstained with hematoxylin. Positive cells were stained dark brown in the nuclei and/or cytoplasm, and the staining was graded into three categories: no staining, weak staining, or heavy staining. Positive staining was considered as expression of DPC4. Normal pancreatic ducts, islets of Langerhans, acini, lymphocytes, and stromal fibroblasts showing moderate to strong expression of DPC4 served as positive internal controls for each section.
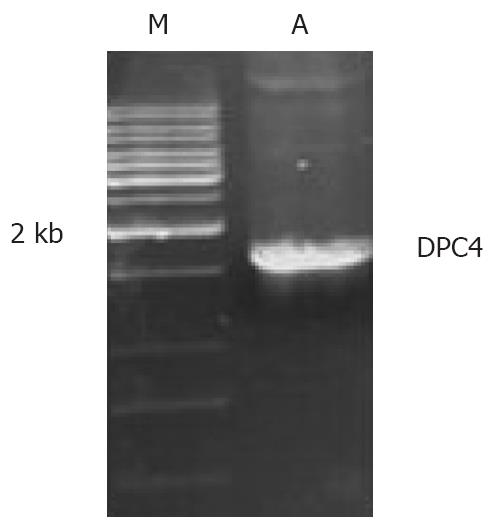
The human DPC4 cDNA was amplified from Smad4/DPC4-pBluescript plasmid (as a gift from Scott Kern) by PCR. The identity of the amplified fragments was confirmed by cycle sequencing using the manufacturer’s directions (Shanghai Sangong Biological Engineering Technology and Servical Co., Ltd), then subcloned to the retroviral vector pLXSN to obtain pLXSN/DPC4+ recombinant with direct insertion, and packaged with GP+E86 and PA317 amphotropic packaging cells. AntiG418 clones were acquired and named as PA317/pLXSN DPC4+ cells. As a control, the empty vector pLXSN also was packaged with GP+E86 and PA317 cells and the antiG418 clones were named as PA317/pLXSN. The virus titer was elevated through cross infection from GP+E86 to PA317 cells and reached 6.0 × 105 pfu/L. DPC4 gene integration in PA317/pLXSN DPC4+ or PA317/pLXSN cells was confirmed by PCR assay.
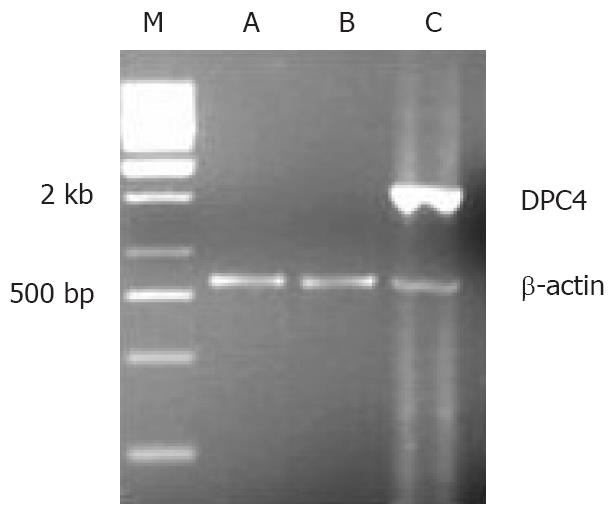
Retroviral supernatant was obtained from the producer cell lines and maintained at 32°C in 5% CO2 atmosphere for 24-48 h. The BxPC-3 lines (purchased from Shanghai Institute for Biological Science, Chinese Academy Science) were transduced using the following protocol. One milliliter of the filtered supernatant was added to 4 × 105 target cells in the presence of 8 μg/mL polybrene. Cells and retroviral supernatant were incubated at 37°C for 4 h. Medium from producer cells was then replaced by RPMI-1640 medium supplemented with 20% fetal calf serum and then in the presence of 2 μg/mL polybrene at 24-h intervals. After the last infection, the daughter cells were subjected to an initial period of selection in 0.2 mg/mL G418 for 4 d and then in 0.5 mg/mL for 1 wk. The positive cells were named as BxPC-3/DPC4. As a positive control, daughter cells transduced by empty vector were named as BxPC-3/pLXSN. As a negative control, mother cells were named as BxPC-3/-.
107 cells were lysed at 4°C in a lysis buffer containing 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride (Sigma Chemical Co., St. Louis, MO, USA), and 25 mmol/L Tris (pH 7.5). The lysates were cleared by centrifugation and boiled for 5 min at 100°C in Laemmli’s SDS-PAGE sample buffer containing 100 mmol/L DTT. Proteins were resolved at 100 V on 10% polyacrylamide gels and transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA, USA). The membranes were blocked with 5% non-fat dry milk, incubated with a Smad4/DPC4 monoclonal antibody (Neo Markers), and then incubated with the secondary clonal antibody. An enhanced chemiluminescence kit (Amersham, Arlington Heights, IL, USA) was used for detection.
Cell growth was determined by the MTT colorimetric growth assay as described previously. Cells were plated in three duplicate wells of a 96-well microtiter plate at 5 × 103 cells/well in 100 μL. After incubation at 37°C in 5% CO2, the cells were visually determined on each of the microtitration plates and 25 mL of RPMI-1640 containing 5 mg/mL of MTT was added to each well. Incubation was continued at 37°C for 3 h. The content of each well was removed, and 200 mL of isopropanol containing 5% 1 mol/L HCl was added to extract the dye. After 30 min of incubation at room temperature and gentle agitation, the Absorbance (A) was measured with a microtitration plate spectrophotometer at 550 nm. The A of the blank, which consisted of an uninoculated plate incubated together with the inoculated plates, was subtracted from the A of the inoculated plates.
A semi-quantitative RT-PCR assay was performed to confirm the expression of vascular endothelial growth factor (VEGF) mRNA. Total RNA was extracted from the three cells, and 1 mg total RNA was reverse-transcribed into first strand cDNA in a reaction primed by oligo (dT) 12-18 primer using Superscript II reverse transcriptase (Invitrogen). Two microliters of the first strand cDNA were used as template for the PCR reactions using Taq polymerase (Life Technologies, Inc.). The PCR reaction started at 94°C for 2 min, followed by 35 cycles (94°C for 30 s, 56°C for 45 s, and 72°C for 45 s), and ended with a 7-min incubation at 72°C. The primers of VEGF were 5'-GGGCCTCCGAAACCATGAACTT-3' and 5'-CGCATCAGGGGCACACAG-3'. The product size was 259 bp. Expression of β-actin was monitored as an internal control; the primers for β-actin were 5'-ACACTGTGCCCATCTACGAGG-3', 5'-AGGGGCCGGACTCGTCATACT-3', and the products was 621 bp. All RT-PCR products were separated by electrophoresis in 1.2% agarose gels and autoradiographed. All experiments were performed in triplicate.
Statistical analysis was performed using the Fisher’s exact probability test and χ2 analysis using the SAS statistical software. A two-tailed Student’s t test was used for statistical analysis of comparative data. Values of P < 0.05 were considered significant.
The RT-PCR assay showed a positive rate of DPC4 mRNA in 100% (36/36) in all normal specimens, compared to 40% (16/40) in adenocarcinoma specimens (n = 76, χ2 = 31.5692, P < 0.0001; Figure 1).
The regional and intense positive expression of DPC4 protein revealed by immunohistochemistry was 10 and four respectively, and all positive rate accounted for 35% (14/40), whereas there was all positive expression in normal tissues (n = 76, χ2 =35.568, P < 0.0001; Figure 2).
The PCR product was about 1.6 kb in the electrophoresis gel as expected (Figure 3). pucm-T/DPC4 gene sequence was identified by Shanghai Sangong Biological Engineering Technology and Servical Co., Ltd., and agreed with our expected result.
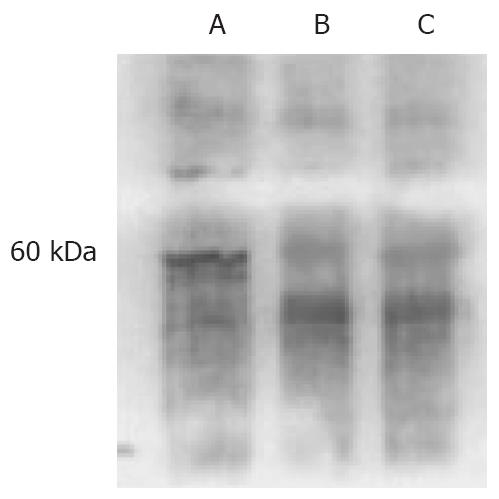
As an internal control, β-actin segments of about 838 bp were obtained in all three cell lines, which indicated that DNA was distilled effectually. DPC4 was expressed only in BxPC-3/DPC4 cells, but not the other control cells, BxPC-3/- and BxPC-3/pLXSN (Figure 4). An approximately 60-kDa protein blot, DPC4 protein, was obtained in BxPC-3/DPC4, but not in the BxPC-3/pLXSN or BxPC-3/- cells (Figure 5).
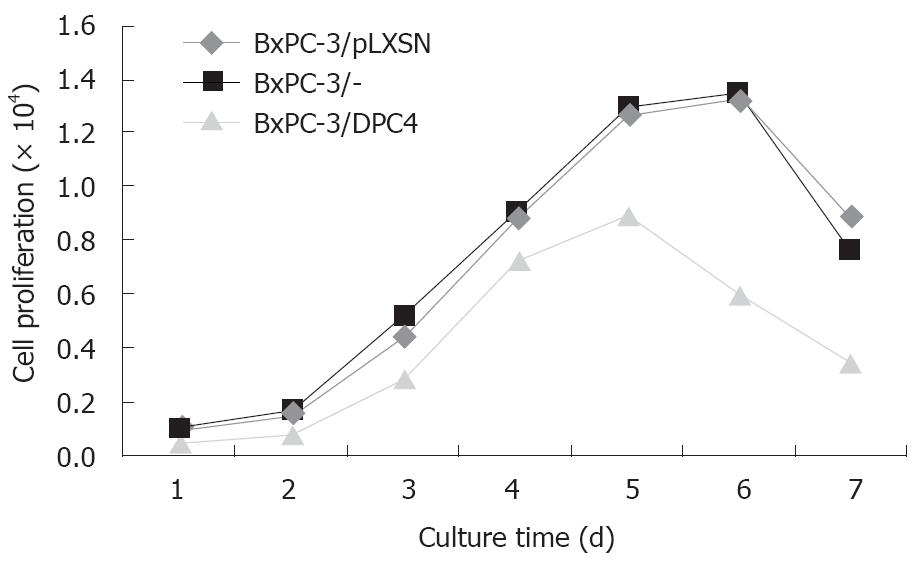
The BxPC-3/DPC4 cells grew much more slowly than the BxPC-3/pLXSN and BxPC-3/- cells. At day 7, the ratio of proliferation inhibition was about 50% (F = 9.65, P = 0.0209, BxPC-3/DPC4 vs BxPC-3/pLXSN; F = 11.03, P = 0.0160, BxPC-3/DPC4 vs BxPC-3/-; Figure 6).
All three cell types had positive expression of VEGF and β-actin. However, semi-quantitative PCR assay showed the level of VEGF mRNA was much lower in BxPC-3/DPC4 than BxPC-3/pLXSN or BxPC-3/- (Table 1).
The majority of patients who present with pancreatic carcinoma have little chance of undergoing operative treatment, and non-operative treatments can offer little survival advantage. It is necessary to look for some other effective treatment for pancreatic carcinoma. TGF-β inhibits cell growth and/or induces apoptosis. In epithelium, disruption of the TGF-β signaling cascade is considered an important mechanism by which tumor cells can escape growth suppression. In a number of cancers, resistance to TGF-β growth inhibition is associated with mutations either in receptor II or in the signal transducers. DPC4/Smad4 belongs to the evolutionarily conserved family of Smad proteins that are linked to the TGF-β superfamily of cytokines, forming a complex with R-Smads in response to ligand stimulation. Disruption of DPC4 can result in a TGF-β signal blackout and is involved in the regulation of cell differentiation, as well as the inhibition of cell proliferation. So, DPC4 is an essential signaling intermediate in the TGF-β receptor-mediated pathway. Abrogation of DPC4 function might cause a breakdown in this signaling pathway and loss of transcription of genes critical to cell-cycle control. Cells might therefore become TGF-β resistant and escape from TGF-β-mediated growth control and thereby contribute to tumorigenesis. C-terminal truncation of DPC4 protein prevents DPC4 homomeric complex formation and heteromeric complex formation with activated Smad2. Furthermore, the mutant protein is unable to be recruited to DNA by transcription factors and hence cannot form transcriptionally active DNA-binding complexes[4]. Otherwise, oligo-ubiquitination positively regulates DPC4 function, whereas poly-ubiquitination primarily occurs in unstable cancer mutants and leads to protein degradation[5].
In the present study the relative contributions of three genes located at the 18q21 region (DCC, Smad2 and DPC4/Smad4) to progression and dissemination of human colorectal and pancreatic tumors were determined. DPC4 inactivation, always accompanied by alteration of all of the other three genes (K-ras, p53, p16)[6], has also been detected in colon, biliary tract, esophageal, gastric, ovarian, head and neck, lung and prostate cancer, especially in pancreatic carcinoma including one mutation and seven homozygous deletions[7-12]. Supporting evidence for the above observation was provided by Takaku and colleagues[13], who constructed knock-out mice with the DPC4 gene. Although DPC4-null mice were embryonically lethal, the heterozygotes of DPC4 were fertile and appeared normal up to the age of 1 year. However, gastric polyps developed in three of 15 heterozygous mice at the age of 50 wk, and in all heterozygous mice at the age of 100 wk. In addition, duodenal polyps were found in mice older than 50 wk. Morphologically, these polyps resembled those of human juvenile polypsis. These results suggest that inactivation of DPC4 is one of the early events in polyp formation in the DPC4 mice, which is analogous to human familial juvenile polyposis. Bartsch et al[14] have also revealed that the expression of DPC4 protein is associated with histopathological grades of pancreatic cancer. Meanwhile, DPC4 inactivation is associated with a poor prognosis[15-19]. Consistent with these reports, DPC4 was shown to be inactivated about in half of pancreatic cancer tissue in our study, whether mRNA or protein. Together with its deletion in pancreas carcinomas, these results suggest that DPC4 has the properties of a tumor suppressor gene, which indicates that it is involved in the carcinogenesis and development of pancreatic carcinoma and is a late event in pancreatic carcinogenesis. The present study can clarify the role of DPC4 in the development of pancreatic carcinoma. In a few substantial studies, significant prognostic markers for pancreatic carcinoma have been reported; markers such as tumor size, lymph node involvement, status of resection margins, DNA ploidy, degree of differentiation, and perineural invasion are inconclusive. In addition, preoperative estimation of tumor size and lymph node involvement is difficult. Deletion of DPC4 in pancreatic carcinoma and loss of DPC4 expression in those patients with poorly differentiated adenocarcinomas was significantly higher than that in those with well and moderately differentiated adenocarcinomas. Therefore, DPC4 gene might preserve phenotypic characteristics under normal conditions and control the malignant progression of pancreatic carcinoma. DPC4 may be proposed as a predictor of prognosis. Recently it was reported that expression of DPC4 can enhance the tumor response to drug treatment[20]. Hoever, it is regretable that we can not draw a consistent conclusion because of deficiency of the detailed clinical and survival data about these pancreatic carcinoma patients in our present study.
Since DPC4 plays a pivotal role in regulating all TGF-β superfamily signal pathways, it is reasonable to postulate that resumption of expression of DPC4 in pancreatic carcinoma cells can inhibit cell proliferation. In fact, DPC4 can induce growth inhibition in breast and colon tumor cells[21,22]. In order to develop an effective therapeutic intervention for patients with pancreatic cancer, we developed a new gene therapy that targets the genetic character of pancreatic cancer, using retroviruses that are selectively replication-competent in tumor cells. The DPC4 transcripts were cloned and subjected to sequence analysis. We performed reconstitution experiments of DPC4 in human pancreatic adenocarcinoma cell line BxPC-3. The wild-type DPC4 DNA was amplified from Smad4/DPC4-pBluescript plasmid by PCR and was enclosed successfully in the retroviral vector pLXSN. The pancreatic carcinoma cells BxPC-3 stably expressing DPC4 were obtained by retroviral transfection of DPC4 expression vectors and by selecting stable clones with G418. Stable transfection of BxPC-3 cells null for DPC4, accompanied by control vectors with DPC4 expression and an empty vector control, yielded similar numbers of G418-resistant clones. RT-PCR and Western blot analysis revealed restored expression of DPC4 in daughter clones derived from expression vector transfection. It was a feasible way to transfer the wild-type DPC4 gene to the DPC4-null cancer cells by pLXSN transfection. Some have reported that the DPC4 expression can inhibit growth of many tumor cells. In breast and colon carcinoma, DPC4 inhibited cell proliferation and induced anoikis[23,24]. Dai et al[25] have explored an inducible system in which DPC4 protein is activated by translocation to the nucleus, when cell lines that stably express wild-type or mutant DPC4 proteins fused to a murine estrogen receptor domain, are treated with 4-hydroxytamoxifen. This induced DPC4-mediated transcriptional activation and a decrease in growth rate, attributable to cell cycle arrest at the G1 phase and induction of apoptosis. In our study, MTT showed that the restored expression of DPC4 in the pancreatic cells can inhibit proliferation by approximately 50% in vitro. These data show that restored expression of functional DPC4 can be efficiently obtained via retrovirus-mediated gene transfer. Supporting our hypothesis, we found that restoration of DPC4 significantly delayed tumor growth in vitro.
The present study indicates that DPC4-inducible apoptosis has the greater consequence in growth control. Indeed, the period of the greatest growth suppression temporally was better correlated with apoptotic responses than with cell cycle arrest. This induced DPC4-mediated transcriptional activation and a decrease in growth rate, attributable to cell cycle arrest at the G1 phase and induction of apoptosis in approximately 55% of pancreatic adenocarcinomas[24,25]. To date, two major apoptotic pathways, the death receptor and the mitochondrial pathway, have been well documented in mammalian cells. However, the involvement of these two apoptotic pathways, particularly the death receptor pathway, in TGF-β1-induced apoptosis is not well understood. Kim et al[21] have reported that apoptosis of human gastric SNU-620 carcinoma cells induced by TGF-β1 is caused by the Fas death pathway, in a Fas-ligand-independent manner, and that the Fas death pathway activated by TGF-β1 is linked to the mitochondrial apoptotic pathway.
Most solid tumor growth is dependent on angiogenesis, and the tumor growth and invasion can be inhibited through anti-angiogenesis. Serum levels of VEGF can decrease significantly after radical resection of the tumor. Elevated preoperative serum VEGF level is a significant prognostic factor, although not independent of stage, for patient survival[26,27]. A decrease in the levels of VEGF could be observed upon restoration of DPC4 expression in cell lines. This effect of DPC4 was found in the present study, implicating DPC4 for the first time as an inhibitor of pancreatic tumor angiogenesis. We found the level of VEGF mRNA level was decreased in the BxPC-3 cells after DPC4 resumption, as demonstrated by the semiquantitative RT-PCR. The retrovirus transfer of DPC4 in DPC4-null cells restored its expression and function, and may be correlated with the suppression of angiogenesis and invasion. However, it is not clear how DPC4 controls VEGF. Other experimental evidence indicates that DPC4 regulates an angiogenic switch by decreasing the expression of VEGF and increasing the levels of angiogenesis inhibitor thrombospondin-1 (TSP-1). It has been reported that DPC4 downregulates VEGF transcription and the secreted matrix metalloproteinase-2 (MMP-2) and MMP-9 expression levels consistently in pancreatic adenocarcinoma cell lines. There was a significant reduction in the tissue immunoreactivity of MMP-2 (a protease activated in angiogenic vasculature), and MMP-9 in samples from mice bearing DPC4-transfected tumors, compared to those from control groups. MMPs have been implicated in primary and metastatic tumor growth and angiogenesis, as well as in tumor invasion and progression. VEGF is a strong inducer and activator of MMP-2, while MMP-9 has been shown to increase the availability of VEGF to its receptors and identifying TSP-1 and VEGF as relevant tumor targets[21].
In summary, our study describes the deletion of tumor suppressor DPC4 in pancreatic carcinoma, and restoration of expression of DPC4 in human cancer cell lines growing in vitro showed the expected results. DPC4 decreased the expression of VEGF. We demonstrated that DPC4 mediates growth inhibition in pancreatic tumour cells even without TGF-β present, and suggest that DPC4 has the potency of a tumor suppressor gene.
Pancreatic carcinoma patients have poor survival, even those who have undergone surgery. The tumor suppressor DPC4 belongs to the evolutionarily conserved family of Smad proteins that are crucial intracellular mediators of signals from TGF-β and is frequently lost in many tumor cells, especially in pancreatic cells. The deletion of DPC4 is involved in the carcinogenesis and development of pancreatic carcinoma. How to further study the role of DPC4 and resumption of the DPC4 gene expression in the PC cell line by the transferring of the vectors containing DPC4 gene in order to inhibit PC growth is becoming a hot topic.
It is necessary to develop a new modality of treatment for pancreatic cancer. Gene therapy strategies may provide therapeutic benefits with a more favorable risk-benefit ratio than the current conventional treatments. With the advances in understanding the pathogenesis, progression, and metastasis of pancreatic carcinoma that have been achieved, studies on gene therapy for pancreatic carcinoma have been attempted in different ways, such as inhibiting oncogenes, and activating tumor suppressor genes. New specific target genes and further development of gene technology may bring a break-through in this field.
We demonstrated that DPC4 can mediate growth inhibition in pancreatic tumor cells even without TGF-β present and reestablish one of the key regulatory controls of cell proliferation. Although numerous attempts have been made and different approaches have been used to identify the target genes, only limited success has been achieved. Our data showed that VEGF may be one of the DPC4-regulated downstream target genes, which will extend our understanding of the mechanism for DPC4 as an inhibitor of pancreatic tumor angiogenesis.
DPC4 is an important tumor suppressor. Further study on the biological nature of DPC4 may contribute to the study of the etiology of pancreatic cancer, and offer a theoretical basis for gene therapy of pancreatic cancer.
In this study the authors demonstrated that deletion of DPC4 in pancreatic carcinoma, and restoring DPC4 expression in pancreatic carcinoma cells could effectively inhibit cancer cell growth in vitro, even without the presence of TGF-β. Re-expression of DPC4 in pancreatic carcinoma cells can downregulate VEGF mRNA expression and anti-angiogenesis therapy may represent a promising therapeutic option.
Peer reviewer: MA Hernandez Bartolome, General Surgery, Hospital Universitario Getafe, Getafe (Madrid) 28904, Spain
S- Editor Zhong XY L- Editor Li M E- Editor Yin DH
| 1. | Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350-353. |
| 2. | Ijichi H, Ikenoue T, Kato N, Mitsuno Y, Togo G, Kato J, Kanai F, Shiratori Y, Omata M. Systematic analysis of the TGF-beta-Smad signaling pathway in gastrointestinal cancer cells. Biochem Biophys Res Commun. 2001;289:350-357. |
| 3. | Bartsch D, Barth P, Bastian D, Ramaswamy A, Gerdes B, Chaloupka B, Deiss Y, Simon B, Schudy A. Higher frequency of DPC4/Smad4 alterations in pancreatic cancer cell lines than in primary pancreatic adenocarcinomas. Cancer Lett. 1999;139:43-49. |
| 4. | Maurice D, Pierreux CE, Howell M, Wilentz RE, Owen MJ, Hill CS. Loss of Smad4 function in pancreatic tumors: C-terminal truncation leads to decreased stability. J Biol Chem. 2001;276:43175-43181. |
| 5. | Moren A, Hellman U, Inada Y, Imamura T, Heldin CH, Moustakas A. Differential ubiquitination defines the functional status of the tumor suppressor Smad4. J Biol Chem. 2003;278:33571-33582. |
| 6. | Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Kloppel G, Kalthoff H. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798-802. |
| 7. | Koyama M, Ito M, Nagai H, Emi M, Moriyama Y. Inactivation of both alleles of the DPC4/SMAD4 gene in advanced colorectal cancers: identification of seven novel somatic mutations in tumors from Japanese patients. Mutat Res. 1999;406:71-77. |
| 8. | Ju HR, Jung U, Sonn CH, Yoon SR, Jeon JH, Yang Y, Lee KN, Choi I. Aberrant signaling of TGF-beta1 by the mutant Smad4 in gastric cancer cells. Cancer Lett. 2003;196:197-206. |
| 9. | Woodford-Richens K, Williamson J, Bevan S, Young J, Leggett B, Frayling I, Thway Y, Hodgson S, Kim JC, Iwama T. Allelic loss at SMAD4 in polyps from juvenile polyposis patients and use of fluorescence in situ hybridization to demonstrate clonal origin of the epithelium. Cancer Res. 2000;60:2477-2482. |
| 10. | Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG, Reiss M. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 2002;62:497-505. |
| 11. | Wang LH, Kim SH, Lee JH, Choi YL, Kim YC, Park TS, Hong YC, Wu CF, Shin YK. Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res. 2007;13:102-110. |
| 12. | Tarafa G, Villanueva A, Farre L, Rodriguez J, Musulen E, Reyes G, Seminago R, Olmedo E, Paules AB, Peinado MA. DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene. 2000;19:546-555. |
| 13. | Takaku K, Miyoshi H, Matsunaga A, Oshima M, Sasaki N, Taketo MM. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59:6113-6117. |
| 14. | Bartsch D, Barth P, Bastian D, Ramaswamy A, Gerdes B, Chaloupka B, Deiss Y, Simon B, Schudy A. Higher frequency of DPC4/Smad4 alterations in pancreatic cancer cell lines than in primary pancreatic adenocarcinomas. Cancer Lett. 1999;139:43-49. |
| 15. | Biankin AV, Morey AL, Lee CS, Kench JG, Biankin SA, Hook HC, Head DR, Hugh TB, Sutherland RL, Henshall SM. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20:4531-4542. |
| 16. | Liu F. SMAD4/DPC4 and pancreatic cancer survival. Commentary re: M. Tascilar et al., The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res., 7: 4115-4121, 2001. Clin Cancer Res. 2001;7:3853-3856. |
| 17. | Maitra A, Molberg K, Albores-Saavedra J, Lindberg G. Loss of Dpc4 expression in colonic adenocarcinomas correlates with the presence of metastatic disease. Am J Pathol. 2000;157:1105-1111. |
| 18. | Isaksson-Mettavainio M, Palmqvist R, Forssell J, Stenling R, Oberg A. SMAD4/DPC4 expression and prognosis in human colorectal cancer. Anticancer Res. 2006;26:507-510. |
| 19. | Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115-4121. |
| 20. | Boulay JL, Mild G, Lowy A, Reuter J, Lagrange M, Terracciano L, Laffer U, Herrmann R, Rochlitz C. SMAD4 is a predictive marker for 5-fluorouracil-based chemotherapy in patients with colorectal cancer. Br J Cancer. 2002;87:630-634. |
| 21. | Kim SG, Jong HS, Kim TY, Lee JW, Kim NK, Hong SH, Bang YJ. Transforming growth factor-beta 1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol Biol Cell. 2004;15:420-434. |
| 22. | Duda DG, Sunamura M, Lefter LP, Furukawa T, Yokoyama T, Yatsuoka T, Abe T, Inoue H, Motoi F, Egawa S. Restoration of SMAD4 by gene therapy reverses the invasive phenotype in pancreatic adenocarcinoma cells. Oncogene. 2003;22:6857-6864. |
| 23. | Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, Luttges J, Kloppel G, Graeven U, Eilert-Micus C. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA. 2000;97:9624-9629. |
| 24. | Ramachandra M, Atencio I, Rahman A, Vaillancourt M, Zou A, Avanzini J, Wills K, Bookstein R, Shabram P. Restoration of transforming growth factor Beta signaling by functional expression of smad4 induces anoikis. Cancer Res. 2002;62:6045-6051. |
| 25. | Dai JL, Bansal RK, Kern SE. G1 cell cycle arrest and apoptosis induction by nuclear Smad4/Dpc4: phenotypes reversed by a tumorigenic mutation. Proc Natl Acad Sci USA. 1999;96:1427-1432. |
| 26. | Karayiannakis AJ, Bolanaki H, Syrigos KN, Asimakopoulos B, Polychronidis A, Anagnostoulis S, Simopoulos C. Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett. 2003;194:119-124. |
| 27. | Khorana AA, Hu YC, Ryan CK, Komorowski RA, Hostetter G, Ahrendt SA. Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg. 2005;9:903-911. |