Published online Oct 28, 2008. doi: 10.3748/wjg.14.6195
Revised: September 16, 2008
Accepted: September 23, 2008
Published online: October 28, 2008
AIM: To evaluate the impact of therapeutic education on adherence to antiviral treatment and sustained virological response (SVR) in a real-life setting in genotype 2/3 hepatitis C, as there are few adherence data in genotype 2/3 infection, even from randomized trials.
METHODS: This prospective survey included genotype 2/3 patients who received peg-interferon alfa-2b and ribavirin. There was no intervention. Adherence was self-reported over the past 4 wk (peg-interferon) or 7 d (ribavirin). Adherence to bitherapy was defined as adherence to the two drugs for ≥ 20 wk. SVR was defined as undetectable RNA ≥ 12wk after the end of treatment.
RESULTS: 370/674 patients received education during the first 3 mo of treatment. After 6 mo, adherence to bitherapy was higher in educated patients (61% vs 47%, P = 0.01). Adherence to peg-interferon was 78% vs 69% (P = 0.06). Adherence to ribavirin was 70% vs 56% (P = 0.006). The SVR (77% vs 70%, P = 0.05) and relapse (10% vs 16%, P = 0.09) rates tended to be improved. After adjustment for baseline differences, education improved adherence [Odds ratio (OR) 1.58, P = 0.04] but not the SVR (OR 1.54, P = 0.06).
CONCLUSION: In genotype 2/3 patients, therapeutic education helped maintain real-life adherence to bitherapy.
- Citation: Cacoub P, Ouzan D, Melin P, Lang JP, Rotily M, Fontanges T, Varastet M, Chousterman M, Marcellin P. Patient education improves adherence to peg-interferon and ribavirin in chronic genotype 2 or 3 hepatitis C virus infection: A prospective, real-life, observational study. World J Gastroenterol 2008; 14(40): 6195-6203
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6195.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6195
| Therapeutic education | |||
| No (n = 304) | Yes (n = 370) | P | |
| Socio-demography | |||
| Men | 170/303 (56) | 229/370 (62) | 0.14 |
| Age (yr) | 44.1 ± 11.4 | 44.9 ± 11.4 | 0.16 |
| Body mass index (kg/m2) | 23.5 ± 4.1 | 24.1 ± 4.2 | 0.06 |
| Employment status | |||
| Professional activity | 190/303 (63) | 220/370 (59) | 0.59 |
| Unemployed | 48/303 (16) | 69/370 (19) | |
| Other | 65/303 (22) | 81/370 (22) | |
| Educational level | |||
| Low | 182/300 (61) | 223/365 (61) | 0.94 |
| High | 118/300 (39) | 142/365 (39) | |
| Origin of incomes | |||
| Paid employment | 166/298 (56) | 183/368 (50) | 0.24 |
| Unemployment incomes | 48/298 (16) | 60/368 (16) | |
| Other | 84/298 (28) | 125/368 (34) | |
| Debts | |||
| Difficult to manage | 8/232 (4) | 27/315 (9) | 0.02 |
| None or easily managed | 224/232 (97) | 288/315 (91) | |
| Comorbidities | |||
| Past psychiatric history | |||
| Depression | 67/303 (22) | 113/370 (31) | 0.01 |
| Suicide attempt | 23/302 (8) | 29/368 (8) | 0.81 |
| Hospitalisation for mental disease | 25/301 (8) | 36/369 (10) | 0.59 |
| Psychiatric disorder | 60/300 (20) | 98/367 (27) | 0.04 |
| Chronic disease | 64/298 (22) | 89/366 (24) | 0.41 |
| Risk factors | |||
| Alcohol consumption > 20 g/d | 10/70 (14) | 24/100(24) | 0.17 |
| Tobacco consumption | 167/299 (56) | 187/366 (51) | 0.24 |
| Drug abuse | |||
| None | 139/303 (46) | 172/368 (47) | 0.02 |
| Former | 158/303 (52) | 174/368 (47) | |
| Current | 6/303 (2) | 22/368 (6) | |
| HCV infection | |||
| Source of HCV infection1 | |||
| Transfusion | 62/304 (20) | 76/370 (21) | 1.00 |
| Injection or intranasal drug abuse | 157/304 (52) | 193/370 (52) | 0.94 |
| Other | 86/304 (29) | 100/370 (27) | 0.86 |
| Duration of HCV infection year) | 20.4 ± 8.4 | 20.0 ± 8.9 | 0.85 |
| Serum HCV-RNA | |||
| ≤ 800 000 IU/mL or equivalent | 121/193 (63) | 162/265 (61) | 0.77 |
| > 800 000 IU/mL or equivalent | 72/193 (37) | 103/265 (39) | |
| HCV genotype | |||
| 2 | 85/304 (28) | 117/370 (32) | 0.31 |
| 3 | 219/304 (72) | 253/370 (68) | 0.31 |
| Coinfection | |||
| Human immunodeficiency virus | 12/303 (4) | 14/369 (4) | 1.00 |
| Hepatitis B virus2 | 3/301 (1) | 6/369 (2) | 0.63 |
| Metavir activity grade or equivalent | |||
| A0 or A1 | 119/226 (53) | 131/272 (48) | 0.32 |
| A2 or A3 | 107/226 (47) | 141/272 (52) | |
| Metavir fibrosis stage or equivalent | |||
| F0 or F1 | 110/227 (49) | 109/272 (40) | 0.04 |
| F2 or F3 | 82/227 (36) | 129/272 (47) | |
| F4 | 35/227 (15) | 34/272 (13) | |
| Knodell score | 7.9 ± 3.0 | 8.4 ± 3.5 | 0.19 |
| Previous anti-HCV treatment course | |||
| None | 242/303 (80) | 303/370 (82) | 0.74 |
| One or more | 61/303 (20) | 67/370 (18) | |
| Therapeutic education | |||
| No (n = 304) | Yes (n = 370) | P | |
| Duration of bitherapy (wk)1 | |||
| Planned | 27.7 ± 8.9 | 27.1 ± 8.2 | 0.49 |
| Actual | 28.4 ± 12.7 | 30.3 ± 14.2 | 0.25 |
| Premature discontinuation (< 20 wk) | 44/304 (15) | 41/370 (11) | 0.20 |
| Peginterferon weekly dose (μg/kg) | |||
| Planned | 1.35 ± 0.29 | 1.41 ± 0.22 | 0.01 |
| Actual at 3 mo2 | 1.31 ± 0.30 | 1.38 ± 0.25 | 0.006 |
| Actual at 6 mo2 | 1.18 ± 0.35 | 1.25 ± 0.31 | 0.02 |
| Ribavirin daily dose (mg) | |||
| Planned | 897 ± 147 | 906 ± 154 | 0.67 |
| Actual at 3 mo2 | 871 ± 166 | 885 ± 175 | 0.25 |
| Actual at 6 mo2 | 771 ± 201 | 803 ± 209 | 0.06 |
| Therapeutic education | ||||||
| Univariate analysis | Multivariate analysis | |||||
| No (n = 304) | Yes (n = 370) | P | OR | 95% CI | P | |
| Adherence1 at 3 mo | ||||||
| Bitherapy | 137/218 (63) | 164/250 (66) | 0.56 | 1.04 | 0.69 to 1.56 | 0.87 |
| Peginterferon | 175/218 (80) | 201/250 (80) | 1.00 | 0.94 | 0.57 to 1.53 | 0.79 |
| Ribavirin | 152/218 (70) | 186/250 (74) | 0.30 | 1.13 | 0.72 to 1.77 | 0.59 |
| Adherence1 at 6 mo | ||||||
| Bitherapy | 83/175 (47) | 126/208 (61) | 0.01 | 1.58 | 1.02 to 2.46 | 0.04 |
| Peginterferon | 121/175 (69) | 162/208 (78) | 0.06 | 1.78 | 1.07 to 2.96 | 0.03 |
| Ribavirin | 98/175 (56) | 145/208 (70) | 0.006 | 1.67 | 1.05 to 2.65 | 0.03 |
| Virological response2 | ||||||
| SVR | 171/246 (70) | 230/298 (77) | 0.05 | 1.543 | 0.99 to 2.40 | 0.06 |
| Nonresponse | 37/246 (15) | 37/298 (12) | 0.38 | |||
| Relapse | 38/246 (16) | 31/298 (10) | 0.09 | |||
As pointed out by the WHO[1], poor adherence to treatment is a worldwide issue in all chronic conditions, which results in poor health outcomes and increased health care costs[2,3]. Even in clinical trials, mean adherence rates are low (43%-78%) in chronic conditions[4-6]. In clinical practice, adherence rates of about 50% are usually reported[7].
Adherence to therapy is critical in the treatment of chronic hepatitis C virus (HCV) infection. The current gold standard therapy is a combination of peg-interferon alfa and ribavirin[8,9]. Patients with genotype 1 infection have a 42%-51% likelihood of achieving a sustained virological response (SVR) after 48 wk of therapy; 78%-82% of patients with genotype 2 or 3 infection respond to 24 wk of treatment, whereas patients with genotype 3 infection and high viral load are difficult to treat (< 70% responders)[10,11]. Non-responders to prior standard bitherapy respond to retreatment in 13% of the cases (29% in non-1 genotype), and relapsers in 58.5% of the cases[12]. Therapy requires weekly subcutaneous injections, twice-daily oral dosing and frequent visits, with blood tests. Side effects occur in nearly all patients. As a result, 15%-20% of patients in clinical trials and > 25% in clinical practice discontinue therapy.
In clinical trials, SVR was significantly improved in those patients with HCV genotype 1 infection who received > 80% of their total peg-interferon dose and > 80% of their ribavirin dose for > 80% of the scheduled treatment duration, in comparison with those who failed these adherence criteria[13]. A review of the 2002-2007 literature confirmed that treatment response is influenced not only by HCV genotype and viral load, but also by patient-related factors including adherence[14]. Moreover, optimal HCV healthcare requires further efforts from providers in communicating with patients, as advocated in France by hepatitis C experts and the Health Ministry[15,16], and shown in studies using patient questionnaires in North America[17,18].
We carried out a large survey named CheObs to evaluate adherence to chronic hepatitis C treatment in the real-life setting in France. We observed that some patients received therapeutic education by a third party (other than the investigator), at the discretion of the investigators, during the study period. According to the consensus that efforts to boost treatment adherence improve SVR rates, we performed the present analysis to evaluate the impact of patient education on real-life adherence and response to treatment with peg-interferon alfa-2b and ribavirin. This analysis was carried out in patients with genotype 2/3 HCV infection (one third of the CheObs cohort), as there are few adherence data for these patients even from randomized trials, and because their data were available before those of patients with other genotypes, due to shorter treatment duration.
The prospective, multicenter, CheObs survey was carried out in teaching hospitals, non-teaching hospitals, and private practice offices highly involved in the management of hepatitis C in France, and supervised by a Scientific Committee. Consecutive patients aged ≥ 18 years with chronic hepatitis C were enrolled if initiation of bitherapy with peg-interferon alfa-2b and ribavirin was scheduled. They could be naive for any chronic hepatitis C therapy or non-responders/relapsers to previous therapy. In accordance with French law, the Ethics Committee’s approval was not required as the protocol was strictly observational and usual practice was unchanged. However, all patients gave informed consent to participate.
Included patients saw their physician at a frequency corresponding to the usual practice in the center. The investigator and the patient completed a questionnaire each at inclusion, at the visits occurring approximately every 3 mo during treatment, and at the visit occurring approximately 6 mo after the end of treatment. Patients filled in their questionnaires in the waiting room and either gave it back to the investigator in a sealed envelope or returned it using a prepaid envelope.
The investigators recorded socio-demographic data, history of HCV infection (including previous treatments), risk factors, comorbidities, patient therapeutic education (provided or not), planned/prescribed hepatitis C treatment, modification of treatment during follow-up, concomitant medications, and adverse events. The virological status, documented by qualitative PCR (Amplicor™, Roche) and test date, was recorded at the last visit.
The patient questionnaires concerned adherence to peg-interferon and ribavirin, and the persons involved in the management of their disease (e.g. health professionals of any discipline, patient associations). The following parameters relating to the past 4 wk were recorded to evaluate adherence to peg-interferon: date of injections (or reason for not having an injection), frequency of and reason for taking peg-interferon at a higher/lower dose than prescribed. The following parameters relating to the past 7 d were recorded to evaluate adherence to ribavirin: number of (200 mg) capsules prescribed morning and evening, date of dosing and number of capsules taken in the morning and evening, reasons for missing doses, reasons for and frequency of taking more/less capsules than prescribed.
As for any survey, there was no protocol-specific intervention. Therapeutic education was defined by intervention of a third party (healthcare professionals other than the prescribing physician) and distribution of support documents and educational material during individual sessions. It was provided at the discretion of the physician. No instruction was given related to which patient should be considered or how education should be provided.
Adherence to bitherapy was defined as adherence to both peg-interferon and ribavirin over a sufficient time period, as self-reported by the patients. According to the “80/80/80” criteria previously defined[13], patients were considered to have adhered to peg-interferon if they had received three or four injections during the past 4 wk, and to have adhered to ribavirin if they had taken at least 22 (200 mg) capsules over the past week. Patients were considered to have adhered to bitherapy if they had adhered to the two drugs for at least 20 wk. These rules were defined according to the recommended number of peg-interferon injections (one per week), the recommended ribavirin daily dose (at least 800 mg/d), and the recommended duration of bitherapy in genotype 2/3 HCV infection (24 wk).
SVR was defined as undetectable HCV RNA in the serum 12 wk after the end of treatment or later. This time interval was considered to be sufficient for this evaluation, since relapse after 12 wk of follow-up is rarely (2%) observed whatever the HCV genotype[19], and the dates of the visits and laboratory tests could not be forced in this observational protocol. Non-response was defined as detectable RNA at the end of treatment, and relapse as undetectable RNA at the end of treatment but detectable at a later time point.
Due to the lack of data in the literature, the CheObs sample size calculation was based on real-life adherence observed in chronic conditions other than chronic HCV infection, such as HIV infection[20], diabetes[21], or hypertension[22]. A total of 1537 patients were required to estimate a 50% adherence rate, with a precision of 2.5% and a type I error of 0.05. Assuming 25%-30% of patients were lost to follow-up or discontinued treatment early, approximately 2000 patients were included overall.
The present analysis was carried out in the subset of patients with genotype 2/3 HCV infection from the CheObs cohort. Statistical analysis was conducted using SAS 8.2 (SAS Institute Inc, Cary, NC, USA). Tests were two-sided and type I error was set at 0.05. Descriptive statistics were performed using all available data. Group comparisons were carried out using Kruskal-Wallis or Fisher’s exact tests. The relationships between adherence or virological response and a set of potential explanatory variables were analysed by forward stepwise logistic regressions. These variables included not only those for which groups differed significantly at baseline (P < 0.05), but also those expected to have a significant impact according to the literature.
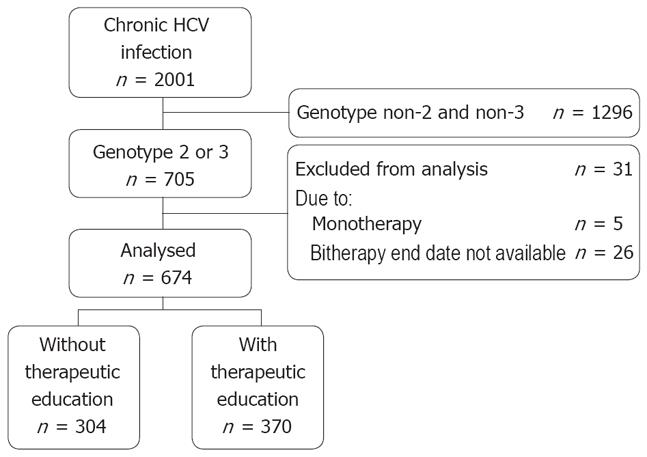
Between 2002 and 2006, 184 investigators enrolled 2001 HCV patients in the CheObs survey, including 705 patients infected with the genotype 2/3 HCV (Figure 1). Of these, 674 patients were analyzed. We observed that 370/674 (55%) patients received therapeutic education during the first 3 mo of treatment and 304 (45%) did not. Among the 82 centers which included the analyzed population, 24 (29%) did not educate any patient, 18 (22%) educated > 0 to 50% of their patients, 19 (23%) educated > 50% to < 100% of their patients, and 21 (26%) educated 100% of their patients. The 31 patients excluded from analysis were similar to the analyzed population for the therapeutic education rate (58%) and all other baseline variables, except for the Metavir activity score, which was more frequently A2/A3 (78% vs 50%, P = 0.009).
In the analyzed population, educated patients had a higher body weight (70.5 ± 14.0 kg vs 67.9 ± 14.3 kg, P = 0.02) than patients without therapeutic education, but a similar body mass index (BMI) (Table 1). They more frequently had a past history of depression (P = 0.01) and current psychiatric disorders (P = 0.04, mainly depression and anxiety), though there was no difference for the nature or proportion of each disorder. Educated patients were also more frequently psychoactive drug users (P = 0.02, mainly cocaine and heroin), but the profile of consumption (injecting behavior and frequency of abuse) was similar. Regarding HCV infection, although educated patients had more frequently significant liver fibrosis than those without therapeutic education (P = 0.04), the proportion of cirrhotic patients was similar (13% and 15%).
A 24-wk bitherapy was scheduled in most patients (571/667, 86%), with no marked difference between groups (Table 2). Treatment for 48 wk was more frequently planned in non-responders/relapsers to previous therapy (43/125, 34%) than in naive patients (49/542, 9%, P < 0.001). The planned weekly dose of peg-interferon was higher in educated patients (P = 0.01), at 1.5 μg/kg per week in most cases (82% vs 75%) whereas patients without therapeutic education were more frequently prescribed lower doses. However there was no difference between the groups for the proportion of retreated patients (Table 1) or that of relapsers/non-responders to previous treatment (P = 0.95). The ribavirin dose prescribed was similar in both groups. It was 800 mg/d in half of the patients (344/669, 51%) and < 800 mg/d in 18 (3%) patients.
The actual duration of bitherapy was shorter than 20 wk in 85/674 (13%) patients (P = 0.20 between groups, Table 2), with an average of 11.1 ± 4.9 wk (median 12) in these patients. Reasons for premature discontinuation were: lost to follow-up (n = 28), safety (n = 27), patient’s request (n = 14), virological (HCV RNA detectable or decrease < 2 log, n = 7), unknown (n = 3), investigator’s request (n = 2), and other (n = 4). They were similar in nature and occurrence (P = 0.79) in both groups. These patients, who were not included in the analysis of virological response, did not differ significantly from those who received a longer bitherapy with regards to the main variables.
Physicians modified the peg-interferon dose less frequently in educated patients (16% vs 22% without therapeutic education, P = 0.046), whereas the ribavirin dose was changed in similar proportions of patients in both groups (17% vs 18%, P = 0.28) nearly always because of adverse effects and weight loss and depression in particular. The difference in the dose between groups remained constant for peg-interferon whereas it increased over time for ribavirin, in particular after the third month of treatment (Table 2). The occurrence of adverse events over the whole study was 82% of patients in each group (P = 0.92).
Overall adherence to bitherapy was 64% (301/468) at 3 mo and 55% (209/383) at 6 mo of treatment. Adherence to peg-interferon (80% and 74% at 3 and 6 mo, respectively) was higher than to ribavirin (72% and 64%, respectively). Patients prescribed high doses of ribavirin (≥ 1000 mg/d) did not differ significantly from those with lower doses with respect to premature treatment discontinuation and adherence to peg-interferon and/or ribavirin.
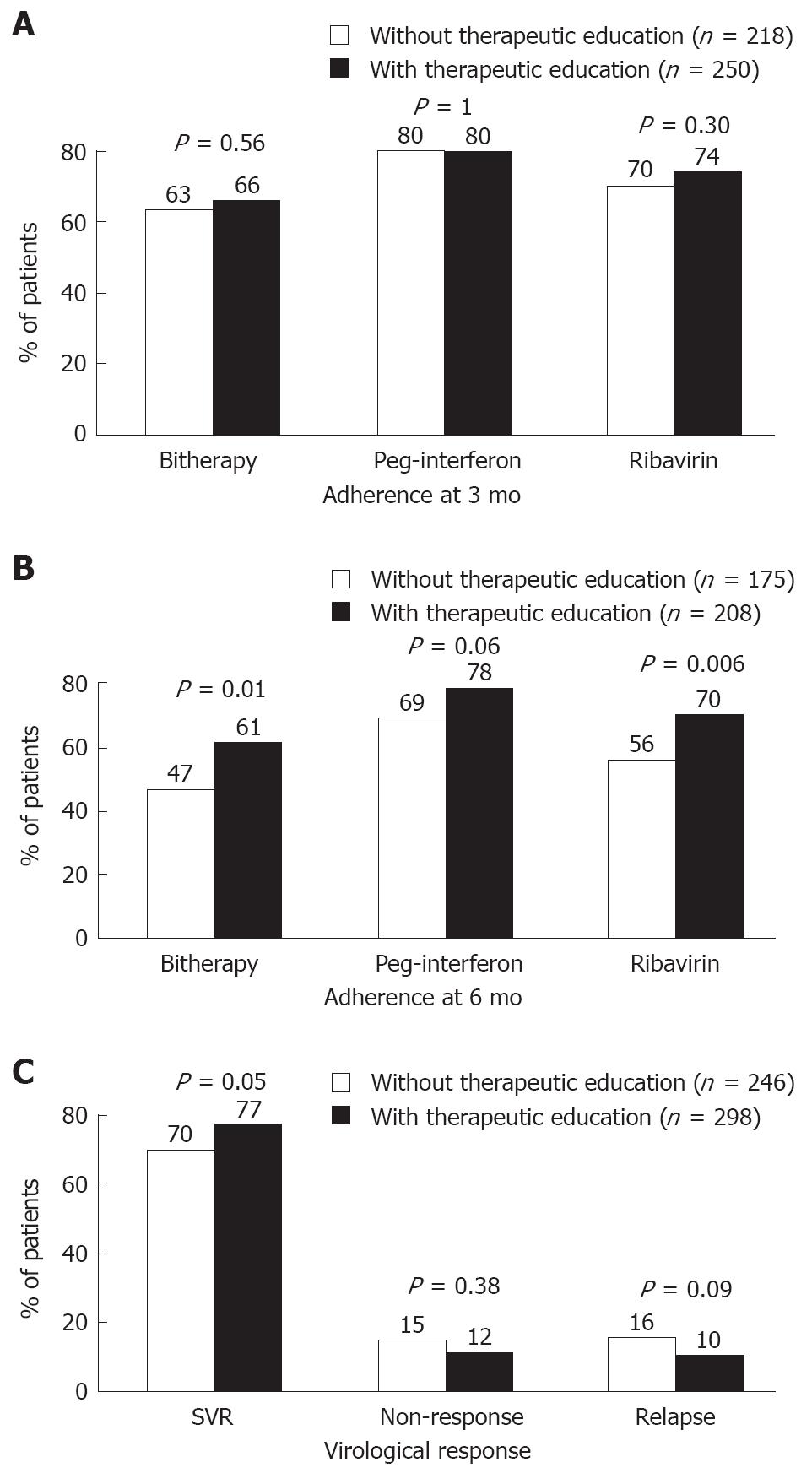
At 3 mo, the proportion of adherents to both drugs was 66% with therapeutic education and 63% without therapeutic education (non-significant difference) (Figure 2 and Table 3). At 6 mo, this proportion was still 61% in educated patients, whereas it dropped down to 47% without therapeutic education (P = 0.01). Multivariate analysis showed that, after adjustment, therapeutic education increased the probability of adhering to bitherapy at 6mo by a factor of 1.58 (95% CI: 1.02 to 2.46).
At 6 mo, the adherence rate was still 78% for peg-interferon and 70% for ribavirin in educated patients, whereas it was reduced to 69% (P = 0.06) and 56% (P = 0.006), respectively, in patients without therapeutic education. After adjustment, therapeutic education increased the probability of adherence by a factor of 1.78 for peg-interferon and 1.67 for ribavirin after 6 mo of bitherapy.
Educated patients more frequently reported contacting persons in the hospital for management of their disease, with a median of three persons vs two for patients without therapeutic education during the first 3 mo of treatment (P=0.016), and three persons vs one during the next 3 mo (P = 0.003). Conversely, patients without therapeutic education reported more frequent contacts with office-based persons, with a median of two persons vs one in educated patients during the first 3 mo (P = 0.012), and two persons vs none during the next 3 mo (P < 0.001).
The overall SVR rate was 74% (401/544). There were 13.6% of non-responders and 12.7% of relapsers. The virological response was better, though not significantly, in educated patients (Figure 2 and Table 3). The SVR rate was higher (77% vs 70%, P = 0.05) and the rate of relapse was lower (10% vs 16%, P = 0.09) in these patients than in patients without therapeutic education. Multivariate analysis confirmed that, after adjustment, the relationship between SVR and therapeutic education was borderline significant (P = 0.06). Response was better in patients whose ribavirin dose was not reduced during the first 3 mo of treatment (P < 0.001), mostly due to an increased SVR rate (362/473, 77% vs 37/67, 55%, P = 0.001) and a decreased rate of non-response (51/473, 11% vs 21/67, 31%, P < 0.001).
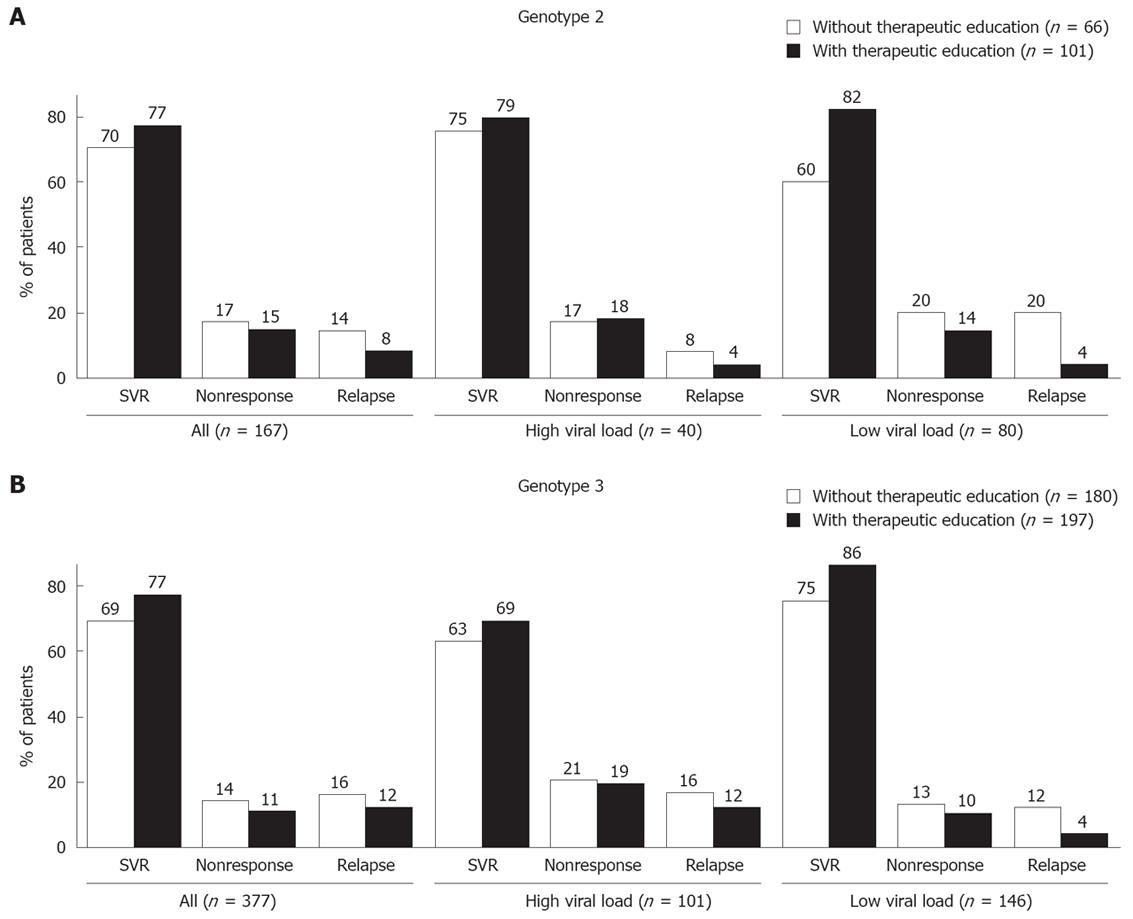
The virological response is shown taking into account genotype and baseline viral load (> or ≤ 800 000 IU/mL) in Figure 3. Though this effect was not significant, therapeutic education was found to have a beneficial impact in all patients. In those with genotype 2 and low viral load, an impact on the SVR (P = 0.038) and relapse (P = 0.047) rates was observed, but the sample size was small (n = 80).
Moreover, although favorable trends were observed, therapeutic education had no significant impact on the virological response in patients treated for the first time (P = 0.27) or in non-responders/relapsers to previous therapy (P = 0.22).
We evaluated the impact of patient therapeutic education by a third party on adherence and virological response to peg-interferon alfa-2b and ribavirin in 674 patients with chronic genotype 2/3 HCV infection. This is the first time that adherence has been evaluated in patients carrying these genotypes, and also the first time that adherence has been assessed in a real-life setting.
Therapeutic education given during the first 3 mo of treatment significantly improved the proportion of patients adhering to bitherapy at 6 mo (OR 1.58, P = 0.01 after adjustment for sex, weight, BMI, educational level, history of depression, psychiatric disorders, alcohol consumption, drug abuse, duration of HCV infection, previous anti-HCV treatment, and peg-interferon dose prescribed at inclusion). The proportion of adherents was stable above 60% until the sixth month of treatment in educated patients, whereas it dropped by more than 10% between the third and sixth month of treatment when there was no therapeutic education. The virological response was also improved in educated patients, with an increased SVR rate (77% vs 70%) and a lower relapse rate (10% vs 16%), though this effect was statistically not significant (P = 0.06 after adjustment for sex, weight, BMI, history of depression, psychiatric disorders, alcohol consumption, drug abuse, duration of HCV infection, previous anti-HCV treatment, HCV genotype, and peg-interferon dose prescribed at inclusion). Since a 12-wk treatment may be very effective in naive genotype 2/3 patients, the fact that adherence was the same over 12 wk may explain why adherence in the later part did not translate to significantly higher SVR rates. As suggested by the borderline P value, this might also be due to the fact that inclusion of one-third of the CheObs cohort in this analysis limited the power to detect statistical significance.
The overall adherence rate to ribavirin (64%-72%) was consistently lower than that to peg-interferon (74%-80%), as expected from their regimen. The ribavirin regimen is somewhat complex (twice-daily oral dosing) whereas peg-interferon alfa-2b adherence is facilitated by the low frequency of administration (weekly injections) and availability of an injecting pen device. Therapeutic education significantly enhanced adherence to ribavirin, which is particularly important for obtaining an SVR[23]. A positive impact was also observed on adherence to peg-interferon, showing that an improvement can still be obtained.
Our results are consistent with the general consensus that patient therapeutic education in clinical practice effectively improves adherence to treatment in chronic disease[2,15,16] and that adherence, even to placebo, is essential to achieve health outcome goals[3]. It should be noted however, that education required the cooperation of specialists and nursing staff, and therefore probably affected both patient-related and health care team-related factors, which are recognized to improve adherence.
The adherence rate to bitherapy (64%) was low compared to the rates of > 70% reported in clinical trials[13]. The SVR rate observed here (72%) was however, in line with that reported in clinical trials (80%) in genotype 2/3 HCV patients treated with similar therapy[24]. Apart from the fact that results are usually more marked in randomized trials, our population included a high proportion of patients with psychiatric disorders and/or drug addicts. This suggests that in real-life situations such comorbidities and high-risk behavior do not have an impact on adherence or response to treatment, so that they may not be as difficult to treat as usually believed.
Unlike during clinical trials, patients in this observational study were not selected or obliged to follow specific procedures and physicians were not instructed on which patient should be considered for therapeutic education. This may be considered to be a weakness of the study, as comparisons were performed on non-randomized groups. The CheObs study was not designed to perform comparisons, but aimed to provide a picture of the real-life setting. The use of multivariate analysis in the present evaluation reduced such bias by taking into account the differences between groups observed at baseline.
The method used to assess adherence may also be criticized. When the study was designed, we chose to use data reported by patients rather than by investigators, to best reflect the real-life situation. However, asking patients to fill a diary each day would have influenced their behavior and led to an unquantifiable overestimation of our primary endpoint. Adherence data were therefore collected over limited time periods. We also chose not to take into account body weight when calculating adherence to ribavirin. Theoretically, we overestimated adherence to ribavirin in patients over 65 kg body weight, i.e. in approximately half the study population at baseline. This bias was however reduced, as weight is known to decrease markedly over time in most treated patients and, as expected, dose reductions occurred for safety reasons in a large proportion of patients (17%-18%) in both groups.
Our results demonstrate that, although patients with HCV genotype 2/3 are those who usually show the best response rates, further efforts may be made to improve outcomes. There is more than one barrier preventing patients from optimal compliance to their treatment regimen[1]. Five interacting dimensions affect adherence: social and economic factors, and factors related to the health care team and system, the condition, treatment, and the patient[2,15,16]. Increasing the impact of interventions aimed at patient-related factors and/or health care team-related factors is essential. Methods that have been shown to be effective in improving therapy include: educational interventions involving patients[25,26]; strategies to improve dosing schedules[6]; interventions that enlist ancillary health care providers such as pharmacists, behavioral specialists, and nursing staff[27,28]; and enhancing communication between physicians and patients[29-31].
To conclude, in the real-life setting, therapeutic education helped maintain adherence to bitherapy in patients with genotype 2/3 infection. There was a trend for a benefit on virological response. This analysis of the real-life impact of patient education by a third party on health outcomes may help to further improve patient quality of life and outcome. The importance of adherence and the role of education should now be studied in a randomized controlled trial in genotype 1/4 infection.
Adherence to therapy is critical in the treatment of chronic hepatitis C virus (HCV) infection. The current gold standard therapy is a combination of peg-interferon alfa and ribavirin. Patients with genotype 2 or 3 infection have a 78%-82% likelihood of achieving a sustained virological response (SVR) after 24 wk of therapy, whereas patients with genotype 3 infection and high viral load are difficult to treat (< 70% responders). Therapy requires weekly subcutaneous injections, twice-daily oral dosing and frequent visits, with blood tests. Side effects occur in nearly all patients. As a result, 15%-20% of patients in clinical trials and over 25% in clinical practice discontinue therapy.
In clinical trials, the SVR rate was significantly improved in those patients with HCV genotype 1 infection who received > 80% of their total peg-interferon dose and > 80% of their ribavirin dose for > 80% of the scheduled treatment duration. A review of the 2002-2007 literature confirmed that treatment response is influenced not only by HCV genotype and viral load, but also by patient-related factors including adherence.
The authors evaluated, perhaps for the first time, the impact of patient therapeutic education by a third party on adherence and virological response to peg-interferon alfa-2b and ribavirin. The analysis was carried out in the 674 patients with chronic genotype 2/3 HCV infection from the CheObs cohort. Therapeutic education given during the first 3 mo of treatment significantly improved the proportion of patients adhering to bitherapy at 6 mo (odds ratio 1.58). Though not significantly, the virological response was also improved in educated patients, with an increased SVR rate (77% vs 70%) and a lower relapse rate (10% vs 16%). Therapeutic education significantly enhanced adherence to ribavirin, which is particularly important for obtaining a SVR. A positive impact was also observed on adherence to peg-interferon, showing that an improvement can still be obtained.
This analysis of the real-life impact of patient therapeutic education by a third party on health outcomes may help to further improve patient quality of life and outcome. Five interacting dimensions affect adherence: social and economic factors, and factors related to the health care team and system, the condition, treatment, and the patient. Methods that have been shown to be effective in improving therapy include: educational interventions involving patients; strategies to improve dosing schedules; interventions that enlist ancillary health care providers such as pharmacists, behavioral specialists, and nursing staff; and enhancing communication between physicians and patients.
This is a subgroup analysis of a bigger project. Since the a priori power calculation was based on 2000 subjects for meaningful statistical analysis, the inclusion of only 630 or so patients in this study clearly limits the power to detect statistical significance.
Peer reviewer: Raymund Rabe Razonable, PhD, Division of Infectious Diseases, Mayo Clinic Institution, 200 First Street SW, Rochester 55905, United States
S- Editor Li DL L- Editor Negro F E- Editor Lin YP
| 1. | Sabate E; Adherence to long-term therapies. Evidence for action. Geneva: World Health Organization; 2003. Available from URL: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. |
| 2. | Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497. |
| 3. | Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. |
| 4. | Cramer J, Rosenheck R, Kirk G, Krol W, Krystal J. Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health. 2003;6:566-573. |
| 5. | Waeber B, Leonetti G, Kolloch R, McInnes GT. Compliance with aspirin or placebo in the Hypertension Optimal Treatment (HOT) study. J Hypertens. 1999;17:1041-1045. |
| 6. | Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296-1310. |
| 7. | Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54 Suppl 1:S57-S60. |
| 8. | Camma C, Licata A, Cabibbo G, Latteri F, Craxi A. Treatment of hepatitis C: critical appraisal of the evidence. Expert Opin Pharmacother. 2005;6:399-408. |
| 9. | Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350-1359. |
| 10. | von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, Bergk A, Bernsmeier C, Haussinger D, Herrmann E. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129:522-527. |
| 11. | Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, Sarrazin C, Harvey J, Brass C, Albrecht J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993-999. |
| 12. | Moucari R, Ripault MP, Oules V, Martinot-Peignoux M, Asselah T, Boyer N, El Ray A, Cazals-Hatem D, Vidaud D, Valla D. High predictive value of early viral kinetics in retreatment with peginterferon and ribavirin of chronic hepatitis C patients non-responders to standard combination therapy. J Hepatol. 2007;46:596-604. |
| 13. | McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069. |
| 14. | Hughes CA, Shafran SD. Chronic hepatitis C virus management: 2000-2005 update. Ann Pharmacother. 2006;40:74-82. |
| 15. | Consensus conference. Treatment of hepatitis C. Gastroenterol Clin Biol. 2002;26 Spec No 2:B303-B320. |
| 16. | Plan National Hepatites Virales C et B (2002-2005). 2002. Available from URL: http://www.sante.gouv.fr/htm/actu/hepatites/sommaire.htm. |
| 17. | Balfour L, Cooper C, Tasca GA, Kane M, Kowal J, Garber G. Evaluation of health care needs and patient satisfaction among hepatitis C patients treated at a hospital-based, viral hepatitis clinic. Can J Public Health. 2004;95:272-277. |
| 18. | Zickmund S, Hillis SL, Barnett MJ, Ippolito L, LaBrecque DR. Hepatitis C virus-infected patients report communication problems with physicians. Hepatology. 2004;39:999-1007. |
| 19. | Zeuzem S, Heathcote EJ, Shiffman ML, Wright TL, Bain VG, Sherman M, Feinman SV, Fried MW, Rasenack J, Sarrazin C. Twelve weeks of follow-up is sufficient for the determination of sustained virologic response in patients treated with interferon alpha for chronic hepatitis C. J Hepatol. 2003;39:106-111. |
| 20. | Moatti JP, Spire B, Duran S. [A review of socio-behavioural studies on adherence to antiretroviral treatments: beyond biomedical models?]. Rev Epidemiol Sante Publique. 2000;48:182-197. |
| 21. | Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19:279-284. |
| 22. | Rudd P. Compliance with antihypertensive therapy: raising the bar of expectations. Am J Manag Care. 1998;4:957-966. |
| 23. | Mulhall BP, Younossi Z. Impact of adherence on the outcome of antiviral therapy for chronic hepatitis C. J Clin Gastroenterol. 2005;39:S23-S27. |
| 24. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. |
| 25. | Patton K, Meyers J, Lewis BE. Enhancement of compliance among patients with hypertension. Am J Manag Care. 1997;3:1693-1698. |
| 26. | Ran MS, Xiang MZ, Chan CL, Leff J, Simpson P, Huang MS, Shan YH, Li SG. Effectiveness of psychoeducational intervention for rural Chinese families experiencing schizophrenia--a randomised controlled trial. Soc Psychiatry Psychiatr Epidemiol. 2003;38:69-75. |
| 27. | Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;38:CD000011. |
| 28. | Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2003;11:185-198. |
| 29. | Burnier M. Long-term compliance with antihypertensive therapy: another facet of chronotherapeutics in hypertension. Blood Press Monit. 2000;5 Suppl 1:S31-S34. |
| 30. | Misdrahi D, Llorca PM, Lancon C, Bayle FJ. [Compliance in schizophrenia: predictive factors, therapeutical considerations and research implications]. Encephale. 2002;28:266-272. |
| 31. | Ross FM. Patient compliance--whose responsibility? Soc Sci Med. 1991;32:89-94. |