INTRODUCTION
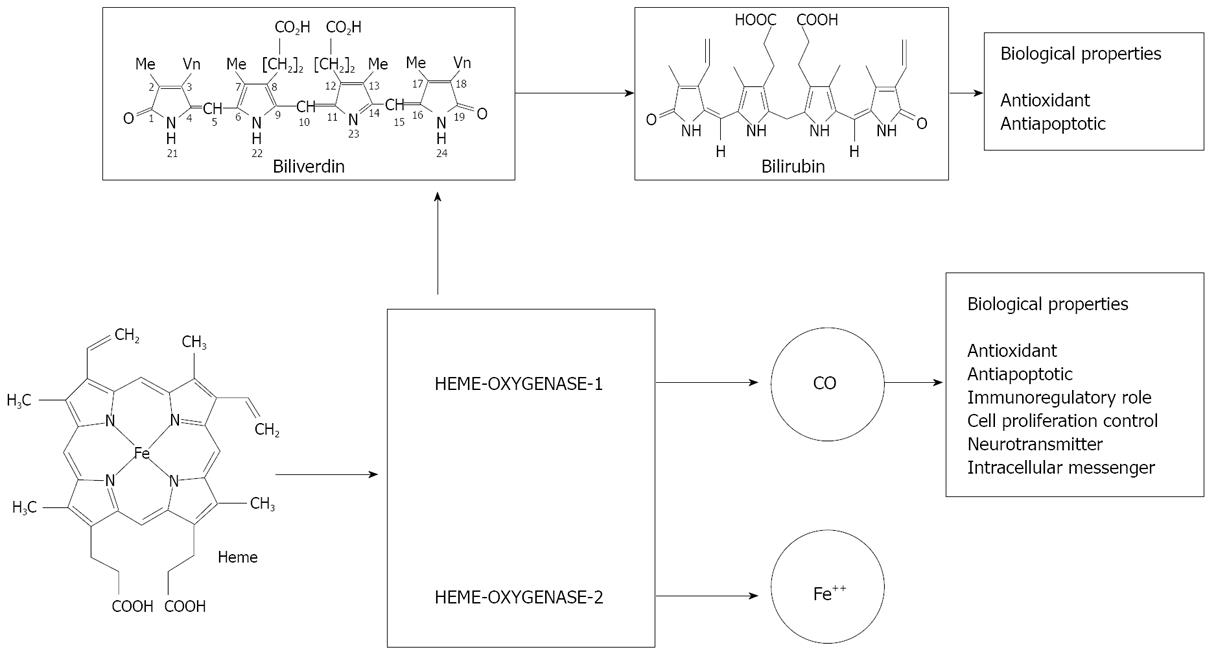
Figure 1 Schematic representation of heme degradation with biological properties of its byproducts.
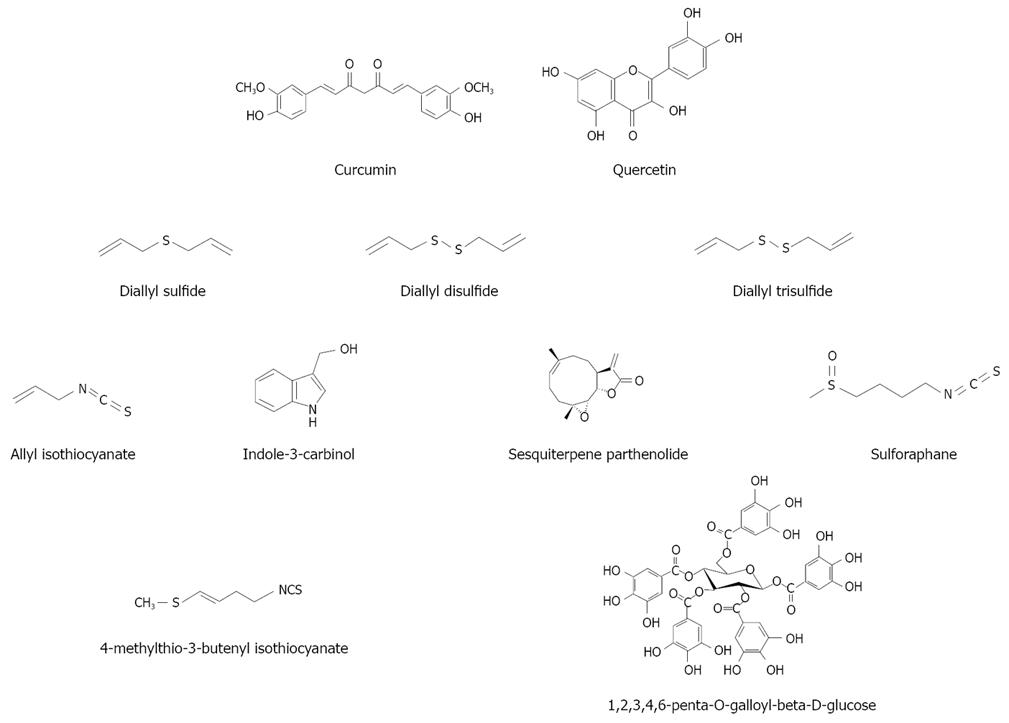
Figure 2 Chemical structures of various natural HO-1 inducers.
Heme oxygenase (HO) is the first, and the rate limiting enzyme in the catabolism of heme[1], to yield equimolar amounts of biliverdin, carbon monoxide (CO) and free iron (Figure 1). To date, two isoforms of HO designated as HO-1 and HO-2 have been identified in mammals[2]. HO-1 is also known as heat shock protein 32. Its human form is composed of 288 amino acids with a molecular mass of 32 800 Da and shares about 80% amino acid sequence identity with rat HO-1[3]. On the other hand, human HO-2 is a 36-kDa protein that consists of 316 amino acids with three cysteine residues[4]. HO-1 is highly inducible by hemin and other chemical and physical agents such as ultraviolet, hydrogen peroxide, heavy metals, hypoxia, and nitric oxide[5,6]. Immunohistochemical studies with specific monoclonal antibodies have revealed the distribution of HO-1 and HO-2 in rat liver with distinct topographical patterns[7]: HO-1 has been shown to be expressed principally in Kupffer cells, while HO-2 is expressed in parenchymal cells[8]. Trakshel et al[2] have demonstrated that under unstimulated conditions, the activity of HO-2 was two- to three-fold higher than that of HO-1, while the activity of HO-1 increased more than 100-fold in the presence of cadmium or cobalt. Under conditions of oxidative stress, hypoxia or hyperthermia, the induction of HO-1 accounts for the majority of heme breakdown, leading to the formation of bilirubin and CO. Since HO-1 is induced as a protective mechanism in response to various stimuli, targeted induction of this stress-response enzyme may be considered as an important therapeutic strategy for the protection against inflammatory processes and oxidative tissue damage (Figure 1). In this article, recent findings on the implications of HO-1 induction on the cellular adaptive cytoprotective response to various insults and inflammatory conditions are reviewed, with particular emphasis placed on targeted HO-1 induction by natural compounds for hepatoprotection.
NATURAL INDUCERS OF HO-1
A number of natural antioxidant compounds contained in foods and plants have been demonstrated to be effective non-stressful and non-cytotoxic inducers of the response protein HO-1 in hepatic cellular models. Most of these compounds are contained in plants that, besides being widely used as food, spices or flavoring, since ancient times, also represent locally traditional medicinal plants.
Curcumin
Curcumin (diferuloylmethane, Figure 2) is the most investigated natural HO-1 inducer. Curcumin is a yellow pigment obtained by populations living in Asian tropical regions by drying and powdering the rhizome of turmeric (Curcuma longa Linn). Widely used as food flavouring, it also plays an important role in traditional medicine because of its anti-inflammatory, anticarcinogenic and antioxidant properties. Curcumin has been demonstrated to be a potent HO-1 inducer in several cellular models (for a Review see Lin[9]). However, the ability of curcumin to induce HO-1 in human hepatocytes has been demonstrated only recently by McNally et al[10]. Interestingly, curcumin is able to confer, at non-toxic doses, a significant protective effect in two transplant-related models of cellular injury, such as cold preservation and warm reperfusion. In a successive study[11], these authors confirmed the HO-1 induction ability of curcumin and elucidated the possible biochemical mechanism. Indeed, at both non-toxic and toxic doses, curcumin treatment resulted in ROS generation, activation of Nrf2 and mitogen-activated protein kinases (MAPKs) and in the inhibition of phosphatase activity. They concluded that at non-toxic doses these multiple pathways converged to induce HO-1.
Flavonoids
Flavonoids are naturally occurring antioxidants belonging to the large family of polyphenols. They are widely distributed in plants used as food, as well as traditional medicines, because of their peculiar variety of clinically relevant properties, such as anti-tumor, antiplatelet, anti-ischemic, and anti-inflammatory activities. Antioxidants with strong free-radical scavenging properties contribute to their biological effects mainly by the Michael reaction acceptor function. However, recent studies[12] have demonstrated the ability of flavonoids to exert their protective properties also by influencing signaling pathways, thus indirectly interacting with the endogenous antioxidative defense system.
Quercetin (Figure 2) is one of the most common flavonoids and, probably, overall the most investigated. In human hepatocytes, quercetin is able to attenuate ethanol-induced oxidative damage by HO-1 induction via p38 and, especially, via ERK/Nrf2 transduction pathway[13,14]. Recently, in the same cellular model, Kluth et al[15] have confirmed that quercetin is able to activate the gene expression regulated by the EpRE of HO-1, although its ability was about 10 times less than that of thyme.
Garlic-derived organosulfur compounds
Diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) (Figure 2), the three major garlic (Allium sativum) organosulfur compounds, have been demonstrated to be HO-1 inducers in hepatic cellular models. In human hepatoma HepG2 cells, Chen et al[16] observed that garlic organosulfur compounds induced HO-1 as the result of Nrf2 activation. Gong et al[17] have confirmed the involvement of Nrf2 activation in the induction of HO-1 by garlic DAS in HepG2 cells. Additionally, in this latter study a different pathway of HO-1 induction has been revealed for DATS, leading the authors to argue that structural differences in terms of the number of sulfur moieties and the length of alkyl side chains can explain the differential effects of garlic-derived organosulfur compounds on the MAPK-mediated activation of Nrf2 and HO-1 induction.
Isothiocyanates
Cruciferous vegetables, particularly Brassica vegetables (i.e. broccoli, Brussels sprouts and cabbage), contain high concentrations of glucosinolates (β-thioglucoside N-hydroxysulfates) that are the precursor of isothio-cyanates, potent inducers of cytoprotective enzymes and inhibitors of carcinogenesis. Noteworthy, the isothiocyanate sulforaphane, due to its peculiar ability to inhibit phase I enzymes and induce phase II enzymes (i.e. HO-1), exerts valuable pleiotropic pharmacologic effects.
Jeong et al[18] have investigated the regulatory role of allyl isothiocyanate, indole-3-carbinol, the sesquiterpene parthenolide and sulforaphane (Figure 2) in the expression and degradation of Nrf2 and the induction of the antioxidant enzyme HO-1. Allyl isothiocyanate is an effective inducer of Nrf2 protein expression, ARE-reporter gene and HO-1, but had little effect on delaying the degradation of Nrf2 protein. Parthenolide and indole-3-carbinol also induced ARE-reporter gene expression and Nrf2, although to a lesser extent if compared to sulforaphane and allyl isothiocyanate. Nonetheless, parthenolide considerably induces the HO-1 expression at a level comparable to sulforaphane, while indole-3-carbinol shows no effect. Of note, sulforaphane strongly induces Nrf2 protein expression and ARE-mediated transcription activation, retards degradation of Nrf2 through inhibiting Keap1, thereby activating the transcriptional expression of HO-1. In the same cellular model, Keum et al[19] have confirmed that transcriptional activation of Nrf2/ARE is critical in sulforaphane-mediated induction of HO-1. Further evidence of the ability of isothiocyanate to activate ARE-mediated HO-1 gene transcription through Nrf2/ARE signaling pathway has been provided by a study from Prawan et al[20] on HepG2-C8 cells.
Hanlon et al[21] have demonstrated that 4-methylthio-3-butenyl isothiocyanate (Figure 2), the isothiocyanate metabolite of glucoraphasatin, a glucosinolate uniquely contained at high concentrations in Spanish black radishes, significantly induces HO-1.
Other compounds and plant extracts
1,2,3,4,6-Penta-O-galloyl-beta-d-glucose (PGG), a bioactive tannin contained in many medicinal plants, is able to induce HO-1 in hepatic cells (Hep-G2)[22]. PGG confers hepatoprotection against oxidative injury by inducing HO-1 expression via stimulating NF-E2-related factor 2 nuclear translocation in an ERK-dependent manner.
In an in vivo study, Yao et al[14] have demonstrated that a standardized Ginko biloba extract, containing not identified terpenes and flavonol heterosides, is able to induce hepatic microsomal HO-1 on mRNA, protein expression and enzymatic activity, providing a hepato-protective effect in ethanol challenged animals.
Hep-G2 cells have been used as a cellular model to demonstrate that the α-methylene-γ-butyrolactone moiety in dehydrocostus lactone, one of the bioactive constituents of the medicinal plant Saussurea lappa, increases cellular resistance to oxidant injury in HepG2 cells, presumably through Nrf2/ARE-dependent HO-1 expression[18]. The same biochemical mechanism is supposed to explain the upregulation of HO-1 synthesis induced by inchinkoto, a Chinese/Japanese herbal medicine[23].
HEME OXYGENASE AS A REGULATOR OF HEPATOBILIARY FUNCTION
In rat liver, HO-1 is prominent in Kupffer cells, whereas HO-2 is most abundant in hepatocytes[7]. Upon stimulation with lipopolysaccharide (LPS), HO-1 not only occurs in tissue macrophages, but is also markedly induced in hepatocytes. On the other hand, HO-2 does not change. Sinusoidal endothelial cells and/or hepatic stellate cells have little if any HO-1, but in culture, appear to express HO-2[8]. HO mRNA levels are high in fetal rat liver during prenatal maturation (9 d before birth) and reach a maximum 24 h after birth, when levels decline but remain above adult levels for at least 1 mo. This correlates with a greater capacity of the liver for bilirubin production in fetuses compared with adults and such circumstances could render the fetus more susceptible to drug injuries because of a depressed heme-cytochrome P450 system[24]. However, Dennery et al[25] have found that serum bilirubin protects against oxidative damage in the first few days of life in neonatal Gunn rats exposed to hypoxia.
Another condition in which there is induction of HO-1 is liver regeneration following 2/3 hepatectomy[26]. In HO-1-deficient mice, there is an increased susceptibility of the liver to endotoxin and interruption of a major pathway of iron recycling[27]. Most importantly, the phenotype of the first human case of HO-1 deficiency includes endothelial cell damage, iron accumulation in the liver and kidney, and an increasing cell susceptibility to heme overloading in vitro[28].
The major source of CO in animals is the degradation of heme by HO; CO produced by HO may serve as an important cellular signal in the microenvironment. In 1994, Suematsu et al[29] have shown that CO is present at submicromolar levels in the liver effluent and that inhibition of HO with ZnPP increases perfusion pressure in the isolated perfused rat liver, an effect that can be reversed by adding CO or a cGMP analogue to the perfusate. CO serves as an endogenous factor that reduces sinusoidal tone involving, at least in part, hepatic stellate cells[30]. The importance of CO as an endogenous modulator of vascular portal perfusion has been confirmed by Pannen et al[31] who demonstrated how NO serves as a potent vasodilator in the hepatic arterial circulation, but exerts only a minor vasodilatory effect in the portal venous vascular bed, while CO does not regulate hepatic artery tone, but is able to maintain portal vascular tone in a relaxed state. Wakabayashi et al[32] have confirmed these results, by showing that the induction of HO-1 with hemin causes a decrease in baseline resistance and in the response to endothelin-1 through an increase in CO production in the extrasinusoidal compartment. We have also shown that overproduction of CO by induction of HO with CoCl2, reduces the response to the vasoconstrictor endothelin-1, but not to phenylephrine, in the isolated perfused rat liver[33].
Elimination of constitutive CO generation through administration of ZnPP not only increases sinusoidal tone, but also stimulates bile-acid-dependent bile flow[34]. The choleretic action coincides with an increase in microvascular tone and oxygen consumption and may thus reflect a prolonged duration of bile acid uptake by hepatocytes. Exogenous CO at micromolar concentrations completely reverses these changes. The effect of ZnPP is mimicked by administration of methylene blue, a soluble guanylate cyclase inhibitor, but not fully reversed by membrane-permeable 8Br-cGMP, suggesting some involvement of cGMP-independent mechanisms. CO generated in hepatocytes may also affect bile excretion by altering the contractility of the bile canaliculus (BC)[34]. Inhibition of CO with ZnPP shortens intervals of contraction of BCs and increases intracellular Ca2+, an effect that is reversed by CO at a micromolar level without increasing cGMP[35]. As to mechanisms of the CO effect, several lines of experimental evidence suggest that CO modulates BC functions through its action on cytochrome P450-mediated calcium mobilization[36].
Jaundice
Bilirubin production is two- to three-fold greater in newborns than in adults. This increase in plasma bilirubin levels is due in large part to the combination of the rapid degradation of fetal hemoglobin in the first few days of life and the immaturity of the hepatic bilirubin conjugating system, thus leading to an increase in unconjugated bilirubin. If the levels of unconjugated bilirubin become too high, the bilirubin may cross the blood-brain barrier, resulting in bilirubin encephalopathy or kernicterus. Phototherapy is the method of choice to lower serum bilirubin levels, but its safety and efficiency have been called into question and is still a matter of debate. The clinical use of HO inhibitors is an alternative therapy. Sn-PP produces a significant decrease in the levels of serum (mean decrease, 38%) and biliary bilirubin (mean decrease, 47%) in normal subjects. The decrease in these parameters lasts for a minimum of 4 d after administration of the metalloporphyrin[37]. The tin porphyrins have been used on newborns with ABO incompatibility[38], on patients with hereditary porphyria[39], liver disease[37], or Crigler-Najjar type I syndrome[40]. Results indicate that the use of SnMP within 24 h of birth in premature newborns substantially moderates the development of hyperbilirubinemia and reduces the requirement for phototherapy markedly (> 75%) in inhibitor-treated infants compared with control subjects[41]. When administered at the appropriate time to near-term and term newborns with hyperbilirubinemia, it can entirely eliminate the need for phototherapy. In patients with biliary cirrhosis and hemochromatosis, Sn-PP is able to reduce bilirubin levels for about 4 d. Biliary bilirubin concentrations decreased (mean decrease, 49%) in hemochromatosis patients after Sn-PP administration. No decrease in biliary bilirubin concentrations can be detected in primary biliary cirrhosis patients under the same conditions[37].
HEME OXYGENASE CONFERS PROTECTION VERSUS DIFFERENT TYPES OF INSULT
Hypoxia, ischemia/reperfusion (IR) and transplantation
Hemorrhagic shock (HS) causes severe hepatic dysfunction or acute failure related to decreased hepatic microcirculatory flow, and results in enhanced hepatic expression of HO-1[8]. Furthermore, the increase in portal resistance, upon blockade of the HO-CO pathway, is much more pronounced after HS compared with sham controls. After HS is endogenously generated, CO preserves sinusoidal perfusion, mitochondrial redox state, and secretory function in the isolated perfused rat liver[42]. This protective role of CO is mediated via a relaxing mechanism, in part, involving Ito cells. Similar results have been obtained by Kyokane et al[43] in endotoxemic rats overexpressing both inducible nitric oxide synthase (i-NOS) and HO-1. In this condition, inhibition of CO, but not of NO, causes marked vasoconstriction and cholestasis. Thus, CO may exert a protective function against hepatobiliary dysfunction after HS and endotoxemia.
Reperfusion injury has been defined as the conversion of reversibly injured cells (myocardial, endothelial, etc.) to irreversibly injured cells, and is mediated by a burst of free-radical generation as the previously hypoxic cells are flooded with oxygen. HO mRNA increases within 4 h of reperfusion of non-necrogenic ischemic rat liver[44]. Redaelli et al[45] have shown that the significant effects of heat preconditioning on liver transplantation after cold storage are prevented by inhibition of HO with tin protoporphyrin, and can be reproduced by administration of cobalt protoporphyrin, an inducer of HO. Thus, overexpression of HO-1 improves post-transplantation survival from 3 d to 3 wk and graft function after prolonged cold ischemia preservation. The mechanism underlying these beneficial effects does not appear to be the prevention of apoptosis. The same beneficial effects of induction of HO-1 with cobalt protoporphyrin (CoPP) or with adenoviral HO-1 (Ad-HO-1) transfection have been shown in steatotic livers[46]. Following cold ischemia/isotransplantation, HO-1 over-expression extended animal survival from 40% in untreated controls to about 80% after CoPP or Ad-HO-1 therapy. This effect is correlated with the preserved hepatic architecture, improves liver function, depresses infiltration by T cells and macrophages, causes suppression of local expression of i-NOS, and modulates the pro- and anti-apoptotic pathways[47]. More recent data have shown that HO-1 modulates pro-inflammatory responses that are triggered via TLR4 signaling, a putative HO-1 repressor[48].
The role of CO in protecting liver grafts from cold I/R injury associated with liver transplantation has been studied by Kaizu et al[49]. Inhalation of CO reduces hepatic injury and is associated with marked downregulation of early mRNA expression for tumor necrosis factor TNF-α, interleukin IL-6, and NOS. CO significantly inhibits phosphorylation of ERK1/2 MAPK and its upstream MEK1/2 and downstream transcriptional factor c-Myc. CO also significantly inhibits I/R injury-induced STAT1 and STAT3 activation. In contrast, CO does not inhibit p38 or JNK MAPK pathways during hepatic I/R injury. These results demonstrate that exogenous CO suppresses early pro-inflammatory and stress-response gene expression and efficiently improves hepatic I/R injury by downregulation of the MEK/ERK1/2 signaling pathway with CO. CO production, evaluated by CO-Hb, is associated with improved function in liver-transplanted patients[50]. Furthermore, an increase in HO-1 during transplantation is more protective than high HO-1 expression before transplantation[51].
Buis et al[52] have shown that donor HO-1 genetic polymorphism may influence the outcome of liver transplantation. Allele genotype is associated with increased graft survival. Graft survival at 1 year is significantly better for A-allele genotype compared to TT-genotype (84% vs 63%). Graft loss, due to primary dysfunction (PDF), occurs more frequently in TT-genotype compared to A-receivers (P = 0.03). Recipients of a liver with TT-genotype have significantly higher serum transaminases after transplantation and hepatic HO-1 mRNA levels are significantly lower compared to the A-allele livers. No differences are found for any outcome variable between class S and LL-variant of the (GT) (n) polymorphism. Haplotype analysis has confirmed dominance of the A (-413) T single nucleotide polymorphism over the (GT) (n) polymorphism. In conclusion, HO-1 genotype is associated with outcome after liver transplantation, suggesting that HO-1 mediates graft survival after liver transplantation. Excessive shear stress secondary to portal hypertension is probably involved in the augmented HO-1 expression in small-for-size graft liver[53]. In this model, recombinant Ad-HO-1 administered to donors 48 h before transplantation enhances HO-1 expression in both whole and small-for-size allografts, with a predominant augmentation in the small-for-size allografts, suggesting favorable conditions for the induction of HO-1 expression in small-for-size allografts. In close relation to the expression level of HO-1, Ad-HO-1 significantly prolongs both whole and small-for size allograft survivals, with a remarkable effect in the small-for-size allograft group. The prolongation of allograft survival is blocked by the HO-1 inhibitor (Zinc protoporphyrin IX). The non-treated small-for-size allografts demonstrate impaired liver function during the early period after reperfusion, which can be improved by over-expression of HO-1, but reversed by the HO-1 inhibitor. The markedly increased expression of HO-1 in small-for-size allografts is associated with lower levels of adhesion molecules and pro-inflammatory cytokines in the early phase after reperfusion[54]. Also, in aged liver, HO-1 overexpression can provide potent protection against cold I/R injury. This effect depends, at least in part, on HO-1-mediated inhibition of the anti-apoptotic mechanism, as an active form of pro-apoptotic caspase-3 (p20) protein, and was found to be 2.9-fold lower at 24 h in the hemin-pretreated group, as compared to saline liver transplant controls[55].
The other product of HO activity, biliverdin, also exerts protective effects against liver I/R injury[56]. Adjunctive biliverdin improves portal venous blood flow from the beginning of reperfusion and increases bile production as compared with the control group. I/R-induced hepatocellular damage, as measured by GOT/GPT release, is diminished by biliverdin. Improved liver function by biliverdin is accompanied by preservation of the histologic structure. Additionally[56], biliverdin adjuvant after orthotopic liver transplantation (OLT) decreases endothelial expression of cellular adhesion molecules (P-selectin and intracellular adhesion molecule 1), and decreases the extent of infiltration by neutrophils and inflammatory macrophages. Biliverdin also inhibits expression of i-NOS and pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) in OLTs. Finally, biliverdin therapy promotes an increased expression of anti-apoptotic molecules independently of HO-1 expression, consistent with biliverdin, being an important mediator through which HO-1 prevents cellular death.
Alcohol and non-alcoholic steatosis
Steato-hepatitis is a liver disease characterized by fat accumulation, inflammation, necrosis, and fibrosis. It can be caused by alcohol, or be independent from alcohol and defined as non-alcoholic steato-hepatitis (NASH). In hepatic NASH, Malaguarnera et al[57] have shown that HO-1 expression is significantly increased, and the increase reflects the severity of the disease. They observed a significant correlation between the increased levels of HO-1 and ferritin, and between the increased levels of HO-1 and lipid peroxidation. Moreover, NASH patients with lower levels of GSH exhibit higher expression of HO-1. Thus, the induction of HO-1 seems an adaptive response against oxidative damage elicited by lipid peroxidation, and it may be critical in the progression of the disease.
The only data on alcoholic steato-hepatitis are those of Yao et al[14] who have shown that the induction of HO-1 by Ginkgo biloba is associated with a decrease in liver damage caused by ethanol feeding for 90 d in rats. This is probably due to the enhanced anti-oxidative capacity against the ethanol-induced oxidative stress and the maintenance of cellular redox balance.
Liu et al[13] have shown that ethanol dose-dependently induces HO-1 and increases HO activity in human hepatocytes in culture, and that HO-1 mRNA increases after 30 min of exposure. Induction of HO-1 with CoPP prevents damage from ethanol. These results have been confirmed by Yao et al[43], who showed that quercetin prevents ethanol toxicity in human hepatocytes, an effect which is mediated by HO-1 induction. HO activity is also increased by chronic ethanol consumption in rats[58]. While 2.5-mo-old rats respond to acute ethanol intoxication by displaying increased expression of liver HO-1 mRNA, and 6-mo-old rats exhibit a mild response, 18-mo-old rats do not show any response, probably because of a decreased transcriptional ability to respond to stress in older animals[59].
Cirrhotic and pre-hepatic portal hypertension
In cirrhosis induced by bile duct ligation, Wei et al[60] have shown that HO-1 mRNA and protein expression is increased in hepatocytes and some Kupffer cells in the early phase of the disease, while HO-2 expression is unchanged. HO-1 induction is also related to iNOS induction.
In cirrhotic livers, mainly biliary cirrhosis, both HO-1 and HO-2 were found to be increased by Goh et al[61]. HO-1 was localized mainly in Kupffer cells, while HO-2 was localized in the cytoplasm of the hepatocytes. Similar results were obtained in patients with post-hepatic cirrhosis by Makino et al[62]. They have shown that HO-1 is increased in the liver, being mainly distributed in Kupffer cells and hepatocytes. By contrast, in livers in which portal hypertension is idiopathic and due to increased perisinusoidal resistance, there is a decreased expression of HO-1 in Kupffer cells and an absence in hepatocytes. A study in cirrhotic patients undergoing liver transplantation has shown that HO-1 is up-regulated through heme-independent stimuli according to the development of portal hypertension and that induced HO-1 plays a pathophysiological role in portal hypertension through CO production[63].
In cirrhotic patients CO-Hb is increased, as demonstrated by Tran et al[64], but does not correlate with disease severity (MELD score, Child Turcotte Pugh score, or other biochemical or clinical measurements). In cirrhotic patients with spontaneous bacterial peritonitis, CO production, evaluated as CO concentration in the exhaled air and blood CO-Hb level, is further increased and may participate in circulatory alterations[65].
A clear role of the HO-CO system in the patho-physiology of hemodynamic alterations related to experimental cirrhotic portal hypertension in the rat is now established.
Decreased HO-2 expression in the liver is associated with increased portal resistance[33], while mesenteric artery dilatation and hypo-reactivity to vasoconstrictors, phenylephrine, KCl, endothelin-1, is associated with induction of HO-1 and increased HO-2. As a confirmation of these findings, transfection of normal rats with human HO-1 mimics mesenteric arterial alterations of portal hypertension[66]. Hyper-expression of HO-1 is particularly relevant in cirrhotic rats with ascites and its function is mediated by large-conductance calcium-activated potassium channels[67]. The alpha subunits of these channels, in particular, are increased in cirrhotic animals and their increase may be mediated by the increased HO-1[68].
In experimental pre-hepatic portal hypertension, obtained by partial portal vein ligation in rats, Fernandez et al[69] have shown that HO activity is increased in the liver. HO-1 expression is present in hepatocytes and Kupffer cells of portal hypertensive rats but not of normal animals, while HO-2 is similarly expressed in all liver cell types of normal and portal-vein ligated rats. They have also evaluated the role of CO in hyporeactivity of the mesenteric vascular beds of prehepatic portal hypertension in rat[69]. In this model, inhibition of HO with ZnMP does not modify the hypo-reactivity to KCl that is partially attenuated by NOS inhibition and completely corrected by simultaneous inhibition of HO and NOS. Also the hypo-reactivity to methoxamine is not affected by ZnMP, but it is completely overcome by L-NAME, without any increase in response after combined inhibition of NOS and HO. In cirrhotic patients with hepatopulmonary syndrome (HPS), characterized by decreased arterial pO2 levels and increased alveolar-arterial oxygen gradient, CO-Hb levels are increased, compared to those without the syndrome, and are correlated with pO2 (P < 0.001) and Aa pO2 (P < 0.001) levels. Thus, CO may contribute to human HPS[70]. These data confirm what was experimentally found: in cirrhosis experimentally induced in the rat by bile duct ligation, NO-mediated up-regulation of HO-1 expression has been shown to participate in HPS[71]. In the same model, HO-1 mRNA transcription and protein expression are significantly increased in cirrhotic hearts compared with sham-operated controls, whereas there is no difference in HO-2 mRNA or protein levels. Total HO activity and cGMP levels are significantly increased in cirrhotic ventricles vs controls, and treatment with ZnPP significantly decreases cGMP production and improves the blunted papillary muscle contractility, whereas it has no effect on control muscles. CO perfusion inhibits papillary muscle contractility, an effect completely blocked by methylene blue and partially blocked by ZnPP. Thus, activation of the HO-CO-cGMP pathway is involved in the pathogenesis of cirrhotic cardiomyopathy[72].
Renal HO-1 expression is decreased in cirrhotic rats (bile ligation) in renal tubules and interlobular arterioles, while it is increased in the liver. The decreased HO-1 is related to renal dysfunction[73].
During HPS caused by liver cirrhosis, pulmonary endothelial NOS expression and NO production are increased. Increased NO contributes to the blunted hypoxic pressure response (HPR) during cirrhosis and may induce HO-1 expression and CO production, exacerbating the blunted HPR. We hypothesized that NO regulates the expression of HO-1 during cirrhosis, contributing to HPS. Cirrhosis was induced in rats by common bile duct ligation (CBDL). Rats were studied 2 wk and 5 wk after CBDL or sham surgery. Lung HO-1 expression was elevated 5 wk after CBDL. Liver HO-1 was increased at 2 wk and remained elevated at 5 wk. In catheterized rats, the blunted HPR was partially restored by HO inhibition. Rats treated with the NOS inhibitor N(G)-nitro-l-arginine methyl ester for the entire 2 wk or 5-wk duration had normalized HO-1 expression and HPR. These data provide in vivo evidence for the NO-mediated up-regulation of HO-1 expression and support the concept that HPS is multifactorial, involving not only NO, but also HO-1 and CO.
In kidneys from CBDL rats, Miyazono et al[73] have shown that HO-1 protein expression is increased slightly at 2 wk but is abolished at 5 wk. In addition, histologically, HO-1 expression was suppressed in renal tubules and interlobular arterioles in 5-wk-old CBDL rats. Conversely, HO-1 expression in liver was strongly increased. Consistent with the development of cirrhosis and renal dysfunction, mean arterial pressure (MAP), glomerular filtration rate (GFR), and renal blood flow (RBF) are decreased in CBDL rats, compared with sham-operated controls. In sham rats, treatment with the selective HO inhibitor ZnPP markedly decreases GFR and RBF to values similar to those measured in CBDL rats without decreasing MAP. In conclusion, decreased renal HO-1 expression contributes to deteriorated renal function and hemodynamics during cirrhosis. This finding provides a novel mechanism for the pathophysiology of renal dysfunction during cirrhosis.
Hepatitis
Interactions between hepatitis viruses B and C and HO have been described, both directly and through the effects on the immune response.
Hepatitis B
Protzer et al[74] have investigated the effects of HO-1 induction in models of human hepatitis B virus (HBV) infection. Adenoviral transfer of an HBV 1.3 genome into wild-type mice was used as a model for acute hepatitis B. HBV transgenic animals were used as a model for chronic HBV infection. To investigate HO-1 effects on HBV replication at a molecular level, stably HBV-transfected hepatoma cells were used. In the acute hepatitis B model, liver injury was reduced significantly after HO-1 induction. In addition, HO-1 showed a pronounced antiviral effect, which was confirmed in stably HBV-transfected hepatoma cells and in persistently HBV replicating transgenic mice. HO-1 induction repressed HBV replication directly in hepatocytes at a post-transcriptional step by reducing stability of HBV core protein and thus blocking refill of nuclear HBV covalently closed circular DNA. Small interfering RNA directed against HO-1 proved that this effect was dependent on the expression level of HO-1. The authors[74] concluded that, besides its hepato-protective effect, HO-1 showed a pronounced antiviral activity in HBV infection.
Hepatitis C
Conflicting data are available on HO-1 in hepatitis C. Ghaziani et al[75] have shown that human hepatoma cells expressing HCV have increased HO-1 and decreased Bach1 expression. Abdalla et al[76], on the contrary, have found a clear decrease in HO-1 and HO-1 mRNA in liver biopsies from HCV-infected patients. The expression of HO-1 was also reduced in cell lines that stably express HCV core protein, which suggests that core gene products are capable of regulating the expression of HO-1. These results are confirmed by Wen et al[77] have who shown that HCV core protein attenuates the induction of HO-1 by heme, heavy metals, and peroxides and contributes to hepatocellular damage by increasing both steady-state levels of pro-oxidants and the susceptibility of hepatocytes to damage by impairing their response to other sources of oxidative stress. Concerning the effects of HO-1 induction on hepatitis C, Shan et al[78] have shown a decrease in HCV replication, an effect similar to that described by Protzer et al[74] in HBV hepatitis.
CRITICAL CONSIDERATIONS AND FUTURE STUDIES
The amount of experimental data that demonstrate important properties of many ingredients and/or bioactive substances from plants and food plants is vast and continues to increase rapidly. The use of terms such as nutraceuticals, functional foods, herbal extracts, bioactive dietary constituents, phytochemicals and similar is becoming copious. In many cases marketing strategies abuse these terms and health properties are claimed although far from being scientifically demonstrated. Thus, researchers are requested to have scientific objectivity in evaluating health properties of food ingredients. It is possible to sustain those diverse bioactive substances from plants and food plants are promising candidates as natural HO-1 hepatic inducers. However, some critical evaluations on literature data are necessary. It is important to note that the majority of studies were conducted in cellular models, whereas only two studies were conducted on rats. Thus, the reproduction of natural HO-1 hepatic inducers in more relevant in vivo models is certainly necessary. With regards to the inductive mechanism of natural HO-1 hepatic inducers, although other pathways cannot be excluded, it seems quite clear that the prevalent mechanism is an ARE-mediated HO-1 gene transcription through the Nrf2/ARE signaling pathway.
Other uncertainties derive from the fact that the referred studies have reported data on natural HO-1 inducers considered both as single chemicals and food extracts. In some cases, scarce or no information has been provided about (1) the quantitative measurements of the proposed active compound; (2) methods of analysis and, (3) extraction procedures. Obviously, the above information is essential to enable other researchers to reproduce the experiments and to obtain comparable data.
When considering a possible therapeutic use of future natural HO-1-inducer-based drugs, the amount of work to perform is even more significant. Indeed, exhaustive information on absorption, distribution, metabolism and excretion by the main possible routes (oral, intraperitoneal, intravenous, intrathecal) are largely insufficient. A potential point of strength of natural HO-1 hepatic inducers is that, generally, they have no toxic effects, and it is presumed that they should not have side effects or teratogenic properties.