Published online Jan 28, 2008. doi: 10.3748/wjg.14.563
Revised: November 8, 2007
Published online: January 28, 2008
AIM: To investigate the role of artificial neural networks in predicting the presence of thyroid disease in atrophic body gastritis patients.
METHODS: A dataset of 29 input variables of 253 atrophic body gastritis patients was applied to artificial neural networks (ANNs) using a data optimisation procedure (standard ANNs, T&T-IS protocol, TWIST protocol). The target variable was the presence of thyroid disease.
RESULTS: Standard ANNs obtained a mean accuracy of 64.4% with a sensitivity of 69% and a specificity of 59.8% in recognizing atrophic body gastritis patients with thyroid disease. The optimization procedures (T&T-IS and TWIST protocol) improved the performance of the recognition task yielding a mean accuracy, sensitivity and specificity of 74.7% and 75.8%, 78.8% and 81.8%, and 70.5% and 69.9%, respectively. The increase of sensitivity of the TWIST protocol was statistically significant compared to T&T-IS.
CONCLUSION: This study suggests that artificial neural networks may be taken into consideration as a potential clinical decision-support tool for identifying ABG patients at risk for harbouring an unknown thyroid disease and thus requiring diagnostic work-up of their thyroid status.
- Citation: Lahner E, Intraligi M, Buscema M, Centanni M, Vannella L, Grossi E, Annibale B. Artificial neural networks in the recognition of the presence of thyroid disease in patients with atrophic body gastritis. World J Gastroenterol 2008; 14(4): 563-568
- URL: https://www.wjgnet.com/1007-9327/full/v14/i4/563.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.563
Atrophic body gastritis (ABG) is characterized by loss of the oxyntic glands and lack of production of chlorhydric acid and intrinsic factor[1], and is epidemiologically and biologically linked to the development of the intestinal-type gastric adenocarcinoma and gastric carcinoid typeI[2]. ABG often presents clinically with pernicious anaemia (PA)[3], but may present also with iron deficiency anaemia and/or long-standing dyspepsia[45].
The association of ABG with thyroid disorders (TD) has firstly been described about 40 years ago[7–9], and the term “thyrogastric disease” has been used to define the presence of thyroid autoantibodies and/or autoimmune thyroid disease in patients with PA[810–12]. These older studies assessed the association between PA and thyroiditis on the basis of gastric and/or thyroid autoantibodies, and found an association ranging from 2.6%-32%[9–12].
Undiagnosed TD may lead to complications related to subclinical hypo- or hyperthyroidism, which may be prevented by an early diagnosis of TD[13]. The diagnosis of TD is generally based on clinical history, assessment of thyroid hormones and autoantibodies as well as ultrasonographic evaluation of the thyroid gland[14]. Albeit, these procedures are non-invasive, a complete diagnostic work-up of TD is time- and cost-consuming. Thus, reliable tools able to select those ABG patients at high risk for harbouring an undiagnosed TD may be useful.
Decision-support systems, based on conventional statistical methods made their entry into medicine several years ago[15], and efforts to improve predictive and prognostic performance of these systems have led to the application of artificial neural networks (ANN) as tools for clinical decision-making[16–18]. ANN are highly flexible computerized mathematical models for understanding and predicting complex and chaotic dynamics in complex biological systems, and have been effectively used to solve non-linear problems related to diagnostic or prognostic queries[1619]. Thus, ANN would appear to be a promising tool for clinical decision-making and have been applied in various areas of medical research[20–22]. In particular, this tool has been previously applied with a high accuracy for predicting atrophic body gastritis on the basis of clinical and serological parameters in patients with anaemia and/or dyspepsia[23]. To our knowledge, studies applying advanced statistical methods in patients with ABG and TD have not yet been performed. This study, therefore, aimed to investigate the role of ANN and linear discriminate analyses (LDA) in predicting the presence of TD in ABG patients.
This study was carried out using a database comprising data from 253 ABG patients (185 females, median age 54 [17-83] years). Of these ABG patients, 123 (48.6%) had pernicious anaemia. In all patients, the presence or absence of TD was evaluated by biochemistry, ultrasonography and endocrinological evaluation at a single tertiary centre. TD was present in 135 (53.4%), and 118 (46.6%) had a healthy thyroid gland. In detail, of the 135 ABG patients with TD, 102 (75.6%) had an autoimmune TD and the remaining 33 (24.4%) had non-autoimmune thyroid disorders, such as a multinodular goitre (n = 16) or a previously diagnosed subacute thyroiditis (n = 10). In addition, 7 patients had undergone complete thyroidectomy prior to the diagnosis of ABG: 3 for thyroid cancer, 3 for large multinodular goitre and 1 for autoimmune thyroiditis.
For each of these 253 patients at the time of diagnosis of ABG, a structured questionnaire was filled out, composed of 29 items concerning anagraphical, life style, family and clinical history, biochemical and histological variables.
Criteria for the diagnosis of ABG: The diagnosis of ABG was based on the presence of fasting gastrin above upper normal values, and histological confirmation of gastric body mucosal atrophy, as previously described[2425]. Briefly, all patients underwent gastroscopy with standardized biopsy sampling from the antrum (n = 3), body (n = 3) for conventional histopathological examination[2425]. The degree of gastritis was assessed according to the updated Sydney System[26]. Atrophy of the body and antral mucosa was defined as focal or complete replacement of oxyntic or pyloric glands by metaplastic pyloric or intestinal glands, respectively[2425]. Furthermore, all patients underwent serological studies: fasting gastrin levels were evaluated by means of a specific radioimmunoassay (RIA) using polyclonal antibody No. 4562[2425]. PepsinogenIlevels were measured using a commercial RIA kit (Pepsik, Sorin, Saluggia, Italy)[25]. Antibodies against anti-parietal cells were assayed using a commercial kit (Autostat, Cogent Diagnostic Ltd, Edinburgh, UK)[2425].
Criteria for diagnosis of thyroid disease: The thyroid status of ABG patients was evaluated on the basis of clinical history, biochemical and ultrasonographic evaluation. Thyroid hormones and thyroid autoantibodies in serum were determined by commercial kits: free triiodothyronine and free thyroxin levels were assayed by radioimmunoassay (Ares-Serono, Milan, Italy); basal thyrotropin levels were assayed by immunoradiometric assay (Radim Techland, Liege, Belgium); antiperoxidase antibodies were measured by a radioligand assay (Radim Techland, Liege, Belgium)[27]. Thyroid gland size, echogenicity of the parenchyma, and nodular lesions were evaluated by ultrasonographic examination. The diagnosis of autoimmune thyroiditis was based on the presence of antiperoxidase antibodies (antibody titres stably > 200 U/mL in at least two separate measurements performed at least 6 mo apart) and characteristic ultrasound features (i.e. non-homogeneous pattern with diffuse reduction of echogenicity) according to Rago et al[14] in presence, but even in absence of mild or overt hypothyroidism[28]. Non-autoimmune thyroid disease (NATD) was assessed on the basis of the presence of ultrasonographic thyroid abnormalities (increased thyroid volume > 25 mL, with the presence of one or more nodules) and the definite absence of antiperoxidase antibodies. Only patients with definite normal morphofunctional and immunological parameters were assumed to have a healthy thyroid gland.
All patients gave written informed consent to the study, which was approved by the local Ethics Committee.
As shown in Table 1, the study was performed on the dataset of 29 input variables of the 253 ABG patients, of which five were continuous, whereas the remaining were dichotomous, as previously described[23]. Biochemical and ultrasonographic data on the diagnosis of TD were not included in the data set. The presence or absence of TD was considered as target variable.
| Input variables | Standard ANN | T&T and IS system | TWIST protocol |
| Positive clinical history for peptic ulcer | X | X | X |
| Active H pylori infection | X | X | X |
| Male | X | X | |
| Female | X | X | X |
| Age at diagnosis of ABG1 | X | X | |
| ABG without associated neoplastic lesions | X | ||
| ABG with associated neoplastic gastric lesions | X | X | X |
| Referred for anemia | X | X | |
| Referred for long-standing dispepsia | X | ||
| Referred for dermatological disorders | X | X | X |
| Referred for endocrinological disorders | X | X | |
| Referred for neurological disorders | X | X | |
| Presence of metaplastic atrophy | X | X | X |
| Presence of multifocal atrophic gastritis | X | X | |
| Actual smoker | X | X | |
| Previous smoker | X | X | |
| Never smoked | X | X | |
| Positive familiy history for gastric cancer | X | ||
| Positive family history for peptic ulcer | X | X | |
| Presence of extrathyroidal autoimmune diseases | X | X | X |
| Presence of extragastric neoplasms | X | X | X |
| Presence of pernicious anemia | X | X | X |
| Presence of iron deficiency anemia | X | X | X |
| Absence of anemia | X | X | X |
| Hemoglobin1 | X | X | X |
| Mean corpuscular volume1 | X | X | X |
| Fasting gastrin1 | X | ||
| Pepsinogen I1 | X | X | X |
| Presence of parietal cell antibodies | X | X | X |
| Total number of input variables used | 29 | 24 | 16 |
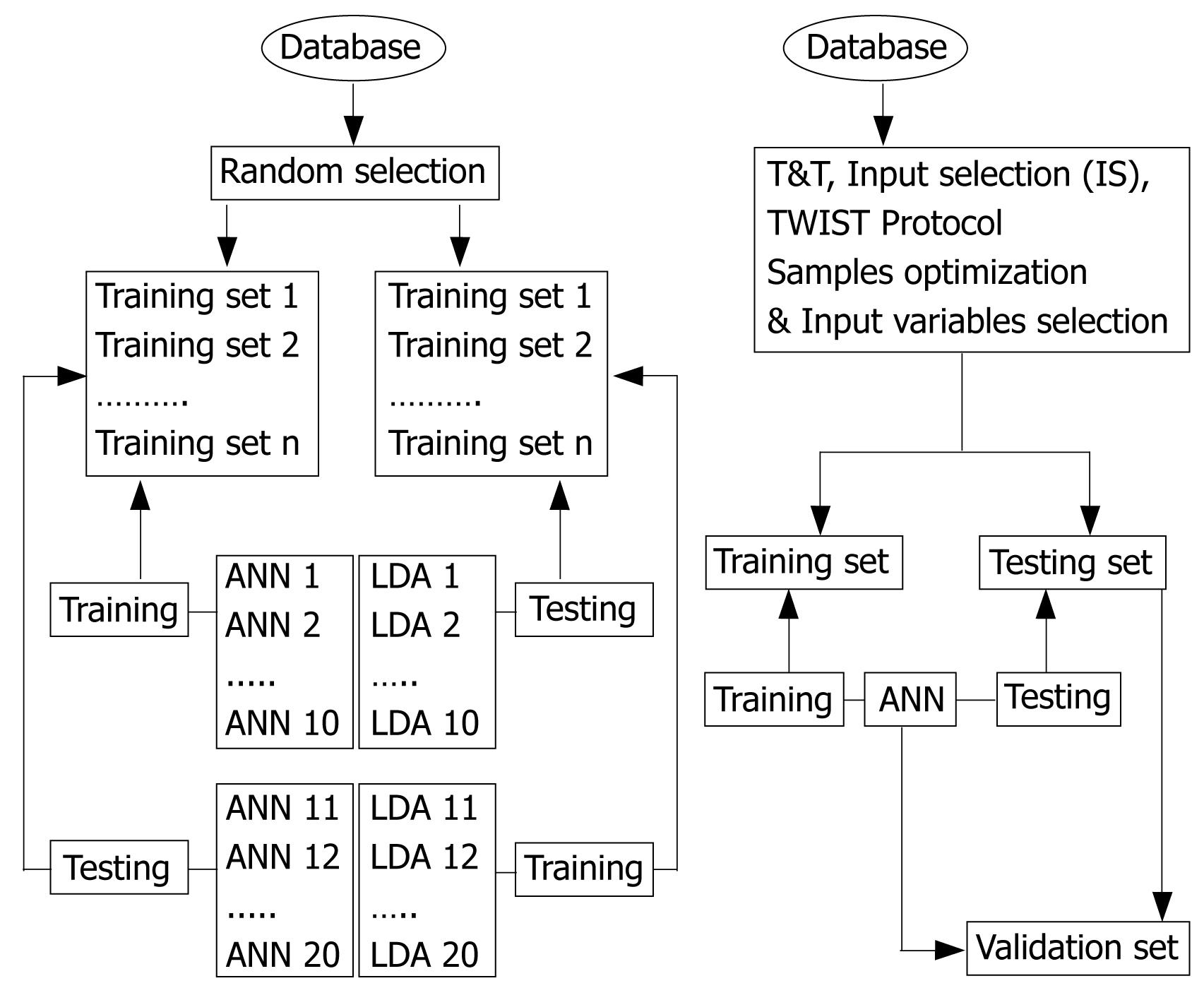
Artificial neural networks (ANN): As shown in Figure 1, the sample of ABG patients was randomly subdivided several times into two equal and balanced sub-samples of subjects with and without TD; one for the training phase (testing) and one for the prediction phase (training). During the training phase, a fixed ANN model is chosen and submitted to the training sub-set in order to learn the linear and non-linear relations existing between all the variables of the original data set and between the input variables and the target variables, performing, in this way, a classification task, i.e., the correct recognition of ABG patients with and without TD. At the end of the training phase, the ANN models showing the best performance on the classification task were submitted to the second sub-sample of patients (the testing set), not previously exposed to the networks, in order to ensure an unbiased evaluation of the classification capability of the ANN. During the so-called “testing phase”, the target variable (presence/absence of TD) has been excluded and the networks made its own evaluation, based on the previously carried out training. In the cross validation protocol, the pairs of sub-set are inverted during the elaboration procedure: each sub-set is used once for the training and once for the testing, this allowing to obtain a non-polarised classification tool.
In order to reduce the number of input variables, selecting those most informative to predict the output, the T&T system, the IS system, as well as the TWIST protocol were used (Table 1). The first system has been developed to solve the problem of establishing the size and assessing the quality of the training and testing sets for a more effective data processing through the ANN models. T&T is a data resampling technique, based on an evolutionary algorithm developed by the Semeion Research Center, the Genetic Doping Algorithm (GenD), able to split the source sample into two or more sub-samples, each one with a similar probability density function. The IS system is an evolutionary wrapper system, also based on the evolutionary algorithm GenD, able to reduce the amount of data, while conserving the largest amount of information available in the dataset. In the TWIST protocol, the T&T and IS systems run in a parallel way to reach a suitable representation of variables and the optimal sample size when dealing with complex and non-linear problems[9]. The software used for the ANN analysis has been developed and were applied by the Semeion Research Center, Rome, Italy.
Linear discriminant analyses (LDA): As shown in Figure 1, the same database was analyzed applying a statistical model based on the assumption of linear correlations between variables, the LDA. In order to optimize the predictive ability, different models were assessed and, as for the ANN procedure, the samples were randomly divided into several sub-samples for the training and testing phases. For the analysis of LDA, the SAS version 6.04 (SAS Institute, Cary, NC, USA) using stepwise procedure was employed. This was performed by the Semeion Research Center, Rome, Italy.
As shown in Table 2, the predictive performance of LDA in discriminating ABG patients with and without TD reached a mean accuracy of 59%, predicting correctly 61% of the ABG patients with TD (sensitivity) and 58% patients with a healthy thyroid gland (specificity). In this model, 82 out of the 135 ABG patients with TD and 69 of the 118 ABG patients without TD were correctly identified. In contrast, the standard ANN model, which used all the 29 input variables, recognized correctly 93 ABG patients with TD and 70 without TD, yielding a mean accuracy, a sensitivity and a specificity of 64%, 69%, and 60%, respectively. Thus, as far as regards the specificity, the predictive performance of LDA and standard ANN were similar, because LDA missed 49 patients and standard ANN 48 (P = 1.00). As far as concerns the sensitivity, i.e. the performance in predicting ABG patients with TD, also in this case, the predictive ability of the standard ANN model was similar to that of LDA with 53 and 42 misclassified patients, respectively (P = 0.20).
In a first step of input data optimization, the sequential application of the T&T and IS systems selected as the most informative 24 of the original 29 independent variables (Table 1). The ANN run with these 24 input variables made less classification errors and thus improved the predictive performance compared to the previous ANN performed on all 29 variables, matching correctly 106 ABG patients with TD and 83 without TD and thus yielding a mean predictive accuracy, a sensitivity and a specificity of 75%, 78%, and 70%, respectively (Table 2).
This ANN model with 24 variables misclassified 35 ABG patients without TD, but the statistical significance with respect to LDA and standard ANN was not reached. In contrast, the predictive performance in identifying correctly ABG patients with TD improved significantly by using the optimized ANN model with 24 variables (P < 0.01) with respect to LDA, reducing the misclassified ABG patients from 49 (LDA) to 29 (ANN 24 variables).
Finally, the application of the TWIST protocol selected as the most informative 16 of the original 29 variables (Table 1). This ANN model reached a better predictive performance compared to the other ANN models, correctly recognizing 82% of the ABG patients with associated TD and 70% of the ABG patients with a healthy thyroid gland; in this case the mean predictive accuracy was 76%, thus outperforming the previous ANN models as well as the traditional linear models in the recognition task. Indeed, the predictive performance in identifying correctly ABG patients with TD improved significantly by using the optimized ANN model with 16 variables (P < 0.001) with respect to LDA, reducing the misclassified ABG patients from 49 (LDA) to 25 (ANN 16 variables). On the other hand, with respect to standard ANN, the predictive performance of the optimized ANN model with 16 variables was improved (P = 0.02).
The results emerging from this study suggest that artificial neural networks are able to predict, with a good accuracy, the presence of TD in ABG patients, by using clinical and gastric biochemical and histological variables. Indeed, the optimised ANN yielded an accuracy of 76%, correctly identifying 82% of ABG patients with TD.
Indeed, the association between ABG and TD is strong, because, as confirmed by the findings of this study, more than half of patients with ABG have an associated TD. Previous studies found an important association up to 30% between pernicious anaemia and TD on the basis of gastric and/or thyroid autoantibodies[3031] and a more recent report assessed the coexistence of ABG and TD on more updated diagnostic criteria[27]. Undiagnosed TD may lead to complications related to subclinical hypo- or hyperthyroidism, which may be prevented by an early diagnosis of TD[13]. Albeit, the diagnostic procedures are generally non-invasive[14] and thus easily accepted by the patients, a complete diagnostic work-up of TD is time and cost consuming. Thus, a computer-based decision-support system, able to select, with an acceptable predictive accuracy, those patients with a high probability of having TD, would enable the thyroid investigations to be better addressed.
Generally, the real databases are composed by a large number of variables that, only apparently, provide the largest possible amount of information. In fact, data collection is performed by trying to include in the database all variables that can have a connection with the problem under investigation; but some of these variables may contain redundant information, which is included also in other variables, or confused information (noise), or may not even contain any significant information at all and be completely irrelevant. If inserted into the dataset, these variables can cause a greater difficulty for the ANN to learn data correctly. Indeed, in this study complex “intelligent” data optimization procedures based on evolutionary algorithms were applied, which permitted to reduce the amount of data, while conserving the largest amount of information available in the dataset, to reach a suitable representation of variables and the optimal sample size when dealing with complex and non-linear problems[29]. This approach, in fact, increased the predictive accuracy as well as the specificity and the sensitivity compared to models performed on non-optimized datasets. Indeed, the reduction in the number of input variables from the initial 29 to 16 led to an increase of the accuracy and the sensitivity of ANN from 64% to 76% and from 69% to 82%, respectively.
It is worthwhile to mention that among the most informative variables for the prediction of TD in ABG patients, PepsinogenI, parietal cell antibodies, female gender and the type of anaemia were included. This finding is not surprising, because all these features have been reported to be related to ABG[21325]. Among variables with the highest predictive power for the presence of TD in ABG patients, the data optimization procedures selected also the presence of active H pylori infection and the positive clinical history for peptic ulcer, which is also related to H pylori infection. This finding is quite interesting, since some authors suggest that the coexistence of ABG and TD may be attributable to common organ-specific antigens that occur as a result of these organs sharing common embryonic origin[32]. However, in general, the presence of a statistical relationship between a predictor variable and the outcome alone does not imply causality[33]. This is even more the case for ANNs-based models, which process data in a non-linear way, and the network logic of prediction cannot be broken down into simple elements of clinical reasoning[3435]. Thus, the variables with the most predictive power selected by the advanced statistical systems should not be viewed as independent predictive variables as perceived by a clinician[3334].
We are aware that the current study had some limits.For the clinical applicability of computer-based decision support systems and their acceptance by physicians, it is necessary to offer a system which is simple, with entry of data that requires little time, thus using a limited number of variables which are readily available to the clinician. In our final optimized ANN model, 16 input variables were selected as most informative for optimizing the prediction of ABG patients with TD. This large amount of input data may thus be a limit for an eventual clinical applicability of this system since the collection of many input variables may be considered time-consuming and scarcely attractive as a clinical decision tool. In any case, this study did not want to emphasize that advanced statistical decision support systems should replace or substitute experienced clinicians, but to underline that, these systems should be viewed as a potential decision aid in order to better address investigations to save costs and to use resources when effectively necessary, as described in other fields of medical settings[36] .
Moreover, we are aware that external validation has not yet been carried out and that, at this stage, the utility and cost benefit of artificial neural networks in recognizing thyroid disease in ABG, as opposed for example to performing thyroid hormones and thyroid autoantibodies and/or thyroid ultrasonographic evaluation, needs to be determined.
In conclusion, this study suggests that artificial neural networks may be taken into consideration as a potential clinical decision-support tool for identifying those ABG patients at risk for harbouring unknown TD and thus requiring diagnostic work-up of their thyroid status.
Atrophic body gastritis (ABG) is characterized by loss of the oxyntic glands and lack of production of chlorhydric acid and intrinsic factor. The association of ABG with thyroid disorders (TD) has firstly been described about 40 years ago, and the term “thyrogastric disease” has been used to define the presence of thyroid autoantibodies and/or autoimmune thyroid disease in patients with ABG, for these reasons thyroid evaluation should be considered in these patients.
Decision-support systems, based on conventional statistical methods made their entry into medicine several years ago, and efforts to improve predictive and prognostic performance of these systems have led to the application of artificial neural networks (ANN) as tools for clinical decision-making. In particular, this tool has been previously applied with a high accuracy for predicting atrophic body gastritis on the basis of clinical and serological parameters in patients with anaemia and/or dyspepsia.
In this article, artificial neural networks are used to investigate their ability in predicting the presence of TD in ABG patients and to evaluate their usefulness in selecting atrophic body gastritis at high risk for harbouring an undiagnosed thyroid disease.
This article suggests that artificial neural networks are able to predict, with a good accuracy the presence of TD in ABG patients, by using clinical, and gastric biochemical and histological variables. Indeed, the optimised ANN yielded an accuracy of 76%, correctly identifying 82% of ABG patients with TD.
The paper seems innovative since, to our knowledge, studies applying advanced statistical methods in patients with ABG and TD have not yet been performed and the obtained results clearly indicated the potential use of this tool in ABG patients at risk for harbouring an unknown thyroid disease and thus requiring diagnostic work-up of their thyroid status.
| 1. | Dixon MF. The Components of gastritis. Histology and pathogenesis. Gastritis. Philadelphia: Lippincott Williams & Williams 1999; 51-66. |
| 2. | Lee EL, Feldman M. Gastritis and other gastropathies. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease: pathophysiology, diagnosis, management. 7th editor. Philadelphia: Saunders 2002; 810-827. |
| 3. | Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441-1448. |
| 4. | Annibale B, Capurso G, Chistolini A, D'Ambra G, DiGiulio E, Monarca B, DelleFave G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439-445. |
| 5. | Annibale B, Lahner E, Negrini R, Baccini F, Bordi C, Monarca B, Delle Fave G. Lack of specific association between gastric autoimmunity hallmarks and clinical presentations of atrophic body gastritis. World J Gastroenterol. 2005;11:5351-5357. |
| 6. | Hershko C, Ronson A, Souroujon M, Maschler I, Heyd J, Patz J. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood. 2006;107:1673-1679. |
| 7. | Doniach D, Roitt IM, Taylor KB. Autoimmune phenomena in pernicious anaemia. Serological overlap with thyroiditis, thyrotoxicosis, and systemic lupus erythematosus. Br Med J. 1963;1:134-139. |
| 8. | Cruchaud A, Juditz E. An analysis of gastric parietal cell antibodies and thyroid cell antibodies in patients with pernicious anaemia and thyroid disorders. Clin Exp Immunol. 1968;3:771-781. |
| 9. | Irvine WJ. Immunoassay of gastric intrinsic factor and the titration of antibody to intrinsic factor. Clin Exp Immunol. 1966;1:99-118. |
| 11. | Feldt-Rasmussen U, Bech K, Bliddal H, Hoier-Madsen M, Jorgensen F, Kappelgaard E, Nielsen H, Lanng Nielsen J, Ryder LP, Thomsen M. Autoantibodies, immune complexes and HLA-D in thyrogastric autoimmunity. Tissue Antigens. 1983;22:342-347. |
| 12. | Carmel R, Spencer CA. Clinical and subclinical thyroid disorders associated with pernicious anemia. Observations on abnormal thyroid-stimulating hormone levels and on a possible association of blood group O with hyperthyroidism. Arch Intern Med. 1982;142:1465-1469. |
| 13. | Weetman AP. Autoimmune Thyroid Disease. In De Groot LJ, Jameson JL, editors. Endocrinology 4th editor. Philadelphia: Saunders 2001; 1409-1421. |
| 14. | Rago T, Chiovato L, Grasso L, Pinchera A, Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dsfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763-769. |
| 15. | Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313:793-799. |
| 16. | Dayhoff JE, DeLeo JM. Artificial neural networks: opening the black box. Cancer. 2001;91:1615-1635. |
| 18. | Wyatt JC. Decision support systems. J R Soc Med. 2000;93:629-633. |
| 19. | Buscema M. Artificial neural networks and complex social systems. I. Theory. Subst Use Misuse. 1998;33:v-xvii, 1-220. |
| 20. | Han M, Snow PB, Brandt JM, Partin AW. Evaluation of artificial neural networks for the prediction of pathologic stage in prostate carcinoma. Cancer. 2001;91:1661-1666. |
| 21. | Das A, Ben-Menachem T, Cooper GS, Chak A, Sivak MV Jr, Gonet JA, Wong RC. Prediction of outcome in acute lower-gastrointestinal haemorrhage based on an artificial neural network: internal and external validation of a predictive model. Lancet. 2003;362:1261-1266. |
| 22. | Selaru FM, Xu Y, Yin J, Zou T, Liu TC, Mori Y, Abraham JM, Sato F, Wang S, Twigg C. Artificial neural networks distinguish among subtypes of neoplastic colorectal lesions. Gastroenterology. 2002;122:606-613. |
| 23. | Lahner E, Grossi E, Intraligi M, Buscema M, Corleto VD, Delle Fave G, Annibale B. Possible contribution of artificial neural networks and linear discriminant analysis in recognition of patients with suspected atrophic body gastritis. World J Gastroenterol. 2005;11:5867-5873. |
| 24. | Annibale B, Marignani M, Azzoni C, D’Ambra G, Caruana P, D’Adda T, Delle Fave G, Bordi C. Atrophic body gastritis: distinct features associated with Helicobacter pylori infection. Helicobacter. 1997;2:57-64. |
| 25. | Marignani M, Delle Fave G, Mecarocci S, Bordi C, Angeletti S, D'Ambra G, Aprile MR, Corleto VD, Monarca B, Annibale B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol. 1999;94:766-772. |
| 26. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 27. | Centanni M, Marignani M, Gargano L, Corleto VD, Casini A, Delle Fave G, Andreoli M, Annibale B. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730. |
| 28. | Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99-107. |
| 29. | Buscema M. A brief overview and introduction to artificial neural networks. Subst Use Misuse. 2002;37:1093-1148. |
| 30. | Cruchaud A, Juditz E. An analysis of gastric parietal cell antibodies and thyroid cell antibodies in patients with pernicious anaemia and thyroid disorders. Clin Exp Immunol. 1968;3:771-781. |
| 31. | Irvine WJ, Cullen DR, Mawhinney H. Natural history of autoimmune achlorhydric atrophic gastritis. A 1-15-year follow-up study. Lancet. 1974;2:482-485. |
| 32. | Venturi S, Donati FM, Venturi A, Venturi M, Grossi L, Guidi A. Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv Clin Path. 4:11-17. |
| 33. | Tu JV. Advantages and disadvantages of using artificial neural networks versus logistic regression for prediction medical outcomes. J Clin Epidemiol. 1996;49:1225-1231. |
| 34. | Dayhoff JE, DeLeo JM. Artificial neural networks: opening the black box. Cancer. 2001;91:1615-1635. |
| 36. | Lisboa PJ. A review of evidence of health benefit from artificial neural networks in medical intervention. Neural Netw. 2002;15:11-39. |