Published online Oct 21, 2008. doi: 10.3748/wjg.14.6100
Revised: May 20, 2008
Accepted: May 27, 2008
Published online: October 21, 2008
A gastric carcinoid tumor concomitant with gastrointestinal stromal tumor (GIST) is rarely encountered in clinical practice. We report a 65-year-old female who had a 0.8 cm gastric carcinoid tumor on the posterior wall of the upper gastric corpus detected during an esophagogastroduodenoscopy at a routine physical examination, and a concomitant 1.1 cm GIST on the anterior wall of the upper gastric corpus incidentally found during surgery of the gastric carcinoid tumor. Normal serum gastrin level and histological findings suggested that she had a type III gastric carcinoid tumor and a GIST which were categorized a very low risk of malignancy, based on their small size and lack of mitosis. Both tumors were treated successfully by surgical excision. The patient had an uneventful recovery. Neither recurrence nor metastasis was found after a 28-mo follow-up.
- Citation: Lin YL, Wei CK, Chiang JK, Chou AL, Chen CW, Tseng CE. Concomitant gastric carcinoid and gastrointestinal stromal tumors: A case report. World J Gastroenterol 2008; 14(39): 6100-6103
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/6100.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6100
Gastrointestinal neuroendocrine tumors are derived from the diffuse neuroendocrine system of the gastrointestinal (GI) tract, composed of amine- and acid-producing cells with different hormonal profiles, depending on their site of origin[1]. Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors arising from interstitial Cajal cells of the wall of the GI tract[2,3]. GISTs can be distinguished from other mesenchymal tumors by optimal immunostaining for CD117, and a prognostic classification is based on tumor size, mitotic score, and MIB-1 grade[4]. Gain-of-function mutation of the c-kit gene, and immunoreactivity of the c-kit protein (CD117) in many GIST support the idea that GIST is a biologically distinct entity. Both carcinoid tumors and GISTs are malignant or potentially malignant tumors, and are considered to have a specific molecular pathogenesis. Herein we report a gastric carcinoid tumor concomitant with a gastric GIST, and also provide a review of the literature.
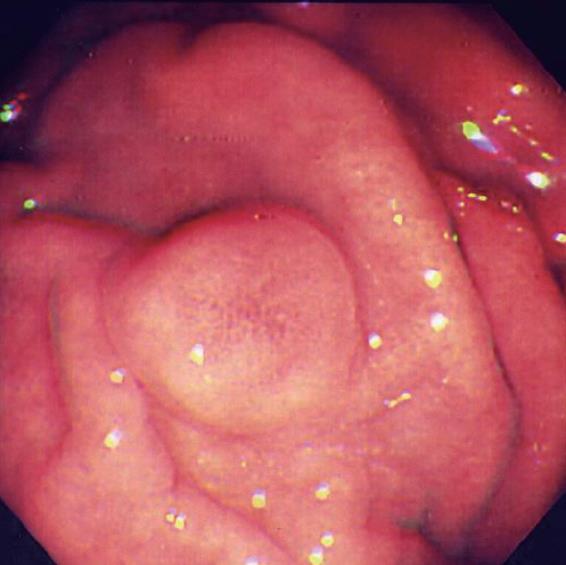
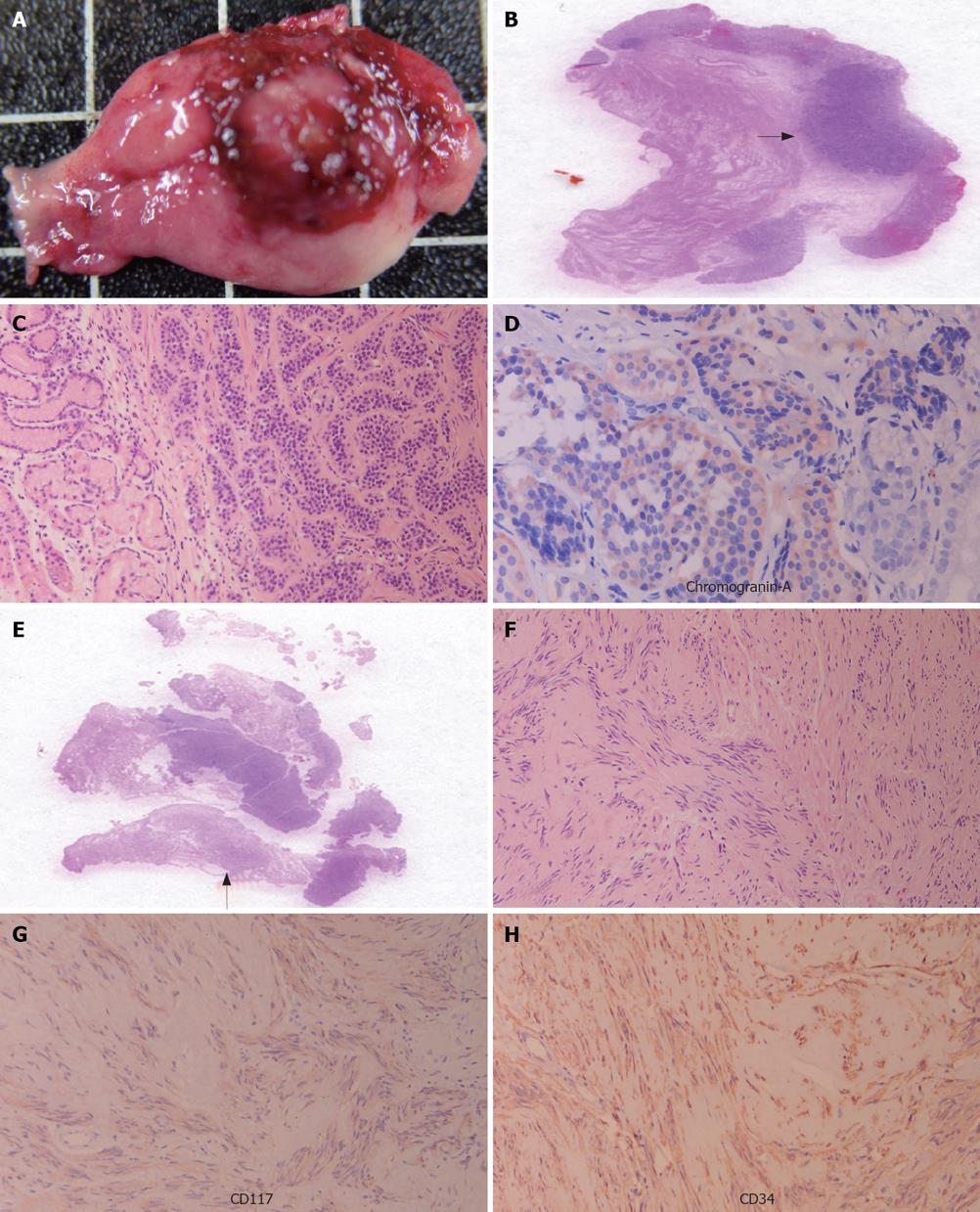
In October 2005, a 65-year-old Asian female came to our hospital for a routine physical examination. She had no history of peptic ulcer, epigastralgia, abdominal pain, diarrhea, flushing, or palpitations. Esophagogastroduodenoscopy showed an approximately 0.8 cm sessile polypoid lesion, with superficial reddish striation, on the posterior wall of the upper gastric corpus (Figure 1). A biopsy sample was taken and eight specimens were acquired. Histological studies showed a gastric mucosa tumor. The tumor demonstrated uniform ovoid cells with cordal and small nestic patterns within the lamina propria. After immunohistochemical (IHC) staining, the tumor cells were positive for cytokeratin, synaptophysin, and chromogranin-A (Figure 2A-D). The Ki-67 index was < 1%. A gastric carcinoid tumor was diagnosed pathologically, and Helicobacter-like microorganisms were also found. A biopsy urease test (CLO test) for Helicobacter pylori (H pylori) infection also demonstrated a positive reaction. The serum gastrin level was 34.4 ng/L (normal range, 25-111 ng/L). Owing to the tumor’s potentially malignant nature, surgery was performed one week later. During the surgical procedure, in addition to the 0.8 cm carcinoid tumor, a 1.1 cm tumor was found incidentally over the serosal side of the anterior wall of the upper gastric corpus. Both small tumors were locally resected simultaneously and separately. Grossly, a patch of gastric tissue, measuring 3.9 cm × 2.5 cm × 0.7 cm, from the posterior wall of the upper corpus disclosed a 0.8 cm × 0.6 cm × 0.3 cm carcinoid tumor. The other gastric tissue taken from the anterior wall of the upper gastric corpus, measuring 2.5 cm × 2.0 cm× 0.5 cm, demonstrated a 1.1 cm × 0.7 cm × 0.3 cm tumor within the muscularis propria of the gastric wall. Histologically, this 1.1 cm tumor displayed swirling bundles of spindle cells with focally palisading areas within the fibrotic stroma. Neither mitotic activity nor tumor necrosis was found. The spindle tumor cells were positive for CD117, CD34, neuron-specific enolase, and S-100 protein, but negative for actin-M851 and glial fibrillary acidic protein after IHC staining (Figure 2E-H). Finally, a gastric carcinoid tumor and a GIST were pathologically diagnosed. The patient had an uneventful recovery, and was discharged one week after surgery. After a 28-mo follow-up, there was no evidence of tumor recurrence or metastasis.
Gastric carcinoid tumor concomitant with gastric GIST is clinically rare. To the best of our knowledge, it has not been reported in the English literature. The pathogenesis of gastric carcinoid tumor concomitant with GIST is unclear. It was reported that H pylori are related to the pathogenesis of gastric carcinoma and mucosa-associated lymphoid tumor[5,6]. We previously reported a case of a 0.4-cm GIST concomitant with an early gastric cancer in 2005[7], and between 2003 and 2007, we intermittently detected one case of multiple 0.2-cm to 0.5-cm gastric neuroendocrine tumors and additional 5 cases of 0.4-15 cm gastric GISTs. All except for 2 of the cases yielded positive CLO tests. The present case also showed positive H pylori infection. However, this finding is more like an incidental event rather than a causal association. Whether the concomitant carcinoid tumor with GIST correlates to H pylori infection or not requires more collected cases and further studies.
Gastric carcinoids are classified into three subtypes, all of which originate from gastric enterochromaffin-like cells in the gastric mucosa. The first subtype is combined with chronic atrophic gastritis (type I). The second subtype, Zollinger-Ellison syndrome, is nearly a part of the multiple endocrine neoplasia-1 (MEN-1) syndrome (type II). Clinically, these two subtypes are linked to a hypergastrinemic state. The third sporadic subtype (type III) occurs without hypergastrinemia but takes an aggressive course, with 54%-66% metastasis[1]. As stated by Shinohara and colleagues, even a 0.5-cm carcinoid tumor can present with metastasis[8]. On account of neither atrophic gastric mucosa nor elevated seral gastrin level in our case, a small type III carcinoid tumor was favored. The potential for metastasis cannot be ignored and demands close follow-up.
Gastric carcinoids may have different clinical features in different locations of GI tract, including abdominal pain, vomiting, and anemia[9]. Carcinoid tumor associated with vascular malformation may cause massive gastric bleeding[10]. Carcinoid syndrome with symptoms of flushing, diarrhea, abdominal pain, cutaneous edema, and bronchoconstriction is uncommon. Due to a small nonfunctional carcinoid, our case never experienced any GI symptom or carcinoid syndrome.
Since 1999, GISTs have been considered to be a group of mesenchymal neoplasms arising from interstitial Cajal cells of the gastrointestinal walls[2,3]. GISTs are now preferentially defined as tumors with c-kit (CD117) positive mesenchymal spindle cells or epithelioid neoplasms, found primarily in the GI tract, omentum, and mesentery[11]. The most important manifestation of this tumor is its indolent, slow-growing nature. This tumor is generally found within the deeper stroma and the submucosa, and incidentally during an imaging study and surgery. In our case, a GIST protruding to the serosal side of the gastric wall was found incidentally during a surgical procedure. Histologically, it arose from the muscularis propria of the gastric wall.
Patients with GIST often present with nonspecific symptoms, such as nausea, vomiting, abdominal pain, GI bleeding, and may have metastatic disease. Bleeding is the most common symptom. The tumor size and mitotic score are considered important diagnostic criteria and prognostic predictive indicators[12]. Our case was asymptomatic and diagnosed as GIST with a very low risk of malignancy based on its small size and lack of mitosis and was positive for CD117 after IHC staining.
Treatment modalities for non-metastatic small carcinoid tumors include endoscopic mucosal resection, minimally invasive laparoscopic wedge resection, and surgery[13,14]. To date, surgery is the mainstay and the only potentially curative therapy for carcinoid tumors. Treatment modalities for metastatic carcinoid tumors include orthotopic liver transplant, hepatic artery embolization, and somatostatin analog, adjuvant indium-111 octreotide-receptor targeted therapy[9]. Therapeutic options for GISTs include surgery and treatment with STI-571 (Gleevec). When inoperative, residual or recurrent tumor exists, STI-571 is the choice[15,16]. Owing to the treatment of two synchronized small gastric tumors in our case, local resections were performed simultaneously and separately. No evidence of tumor recurrence or metastasis was found after a 28-mo follow-up period.
In conclusion, we report a rare case of small gastric carcinoid tumor concomitant with a small gastric GIST with no clinical symptoms and positive H pylori infection. More studies are required for evaluating the relation between H pylori infections and tumorigenesis of concomitant gastric carcinoid and gastric GIST. A long term follow-up period of all carcinoids and GISTs is greatly needed, due to their potential for metastasis.
Peer reviewer: Xin Chen, Professor, University of California, 513 Parnassus Ave. S-816, Box 0912, San Francisco 94143, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Yin DH
| 1. | Jensen RT. Neuroendocrine tumors of the gastrointestinal tract and pancreas. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill 2005; 2220-2226. |
| 2. | Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389. |
| 3. | Robinson TL, Sircar K, Hewlett BR, Chorneyko K, Riddell RH, Huizinga JD. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157-1163. |
| 4. | Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669-676. |
| 5. | Moss SF, Malfertheiner P. Helicobacter and gastric malignancies. Helicobacter. 2007;12 Suppl 1:23-30. |
| 6. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. |
| 7. | Lin YL, Tzeng JE, Wei CK, Lin CW. Small gastrointestinal stromal tumor concomitant with early gastric cancer: a case report. World J Gastroenterol. 2006;12:815-817. |
| 8. | Shinohara T, Ohyama S, Nagano H, Amaoka N, Ohta K, Matsubara T, Yamaguchi T, Yanagisawa A, Kato Y, Muto T. Minute gastric carcinoid tumor with regional lymph node metastasis. Gastric Cancer. 2003;6:262-266. |
| 9. | Leung LK, Ng EKW, Sung JJY. Tumors of the Stomach. Textbook of Gastroenterology. 4th ed. Philadephia: Lipincott, Williams & Wilkins 2003; 1434-1435. |
| 10. | Roncoroni L, Costi R, Canavese G, Violi V, Bordi C. Carcinoid tumor associated with vascular malformation as a cause of massive gastric bleeding. Am J Gastroenterol. 1997;92:2119-2121. |
| 11. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. |
| 12. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339-1352. |
| 13. | Kadikoylu G, Yavasoglu I, Yukselen V, Ozkara E, Bolaman Z. Treatment of solitary gastric carcinoid tumor by endoscopic polypectomy in a patient with pernicious anemia. World J Gastroenterol. 2006;12:4267-4269. |
| 14. | Ishikawa K, Etoh T, Shiromizu A, Inomata M, Shiraishi N, Kashima K, Kitano S. A case of sporadic gastric carcinoid tumor treated successfully by laparoscopy-assisted distal gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:348-350. |
| 15. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. |