Published online Oct 21, 2008. doi: 10.3748/wjg.14.6096
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: October 21, 2008
The molecular targets of sunitinib are receptor tyrosine kinases (RTKs), and this drug has also been known to exert blocking effects on the activation of KIT, which is similar to the mechanism of action of imatinib. Moreover, sunitinib has an additional anti-angiogenic effect through its inhibition of the vascular endothelial growth factor receptor activation. We report here a 70-year-old patient diagnosed with a recurrent gastrointestinal stromal tumor (GIST), which invaded the transverse colon and led to a perforation during sunitinib treatment. A computed tomography scan and 3-dimensional reconstruction showed necrosis of the recurrent hepatic mass and perforation of the invaded transverse colon. After percutaneous drainage of the intraperitoneal abscess, antibiotic treatment and restricted diet, the condition of the patient improved. The present case is the first to report that sunitinib, which is administered to treat GIST resistant to imatinib, can cause unexpected colon perforation and subsequent peritonitis.
- Citation: Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol 2008; 14(39): 6096-6099
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/6096.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6096
Sutent (Pfizer, New York, NY) is the malate salt of sunitinib, which is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs)[1]. This molecular target is the basis for the application of sunitinib in the treatment of gastrointestinal stromal tumors (GISTs), which result from the molecular abnormalities of tyrosine kinases such as KIT[2]. In addition, the targets of sunitinib involve vascular endothelial growth factor receptors (VEGFR1, VEGFR2 and VEGFR3), platelet-derived growth factor receptors (PDGFRα and PDGFRβ) and the like. Although this drug has been approved by the US Food and Drug Administration (FDA) for treatment of GIST patients following progression or resistance to imatinib (Gleevec; Novartis, Switzerland), randomized phase III clinical trials have shown some common adverse effects such as diarrhea, mucositis, abnormal heart function and myelosuppression of imatinib[3]. However, there has never been a report focused on the relationship between bowel perforation and sunitinib treatment. In the present paer, we describe, for the first time, a case of unexpected colon perforation during sunitinib treatment.
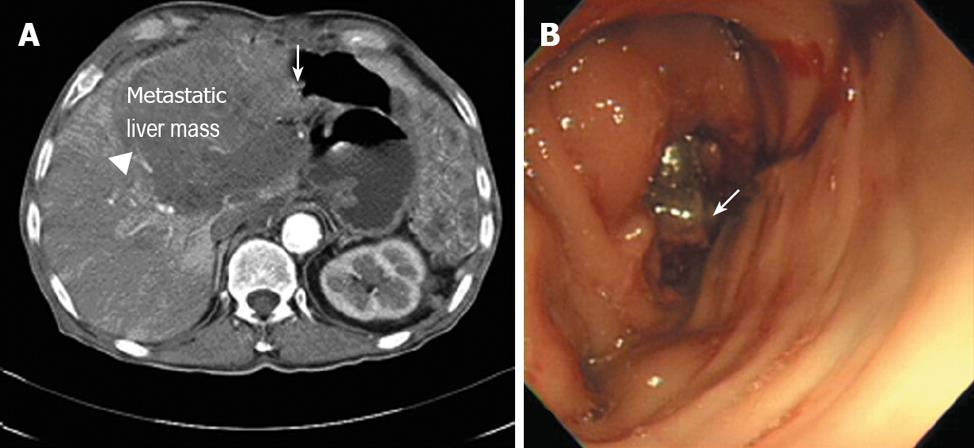
Here we report a 70-year-old patient who was initially treated with proximal gastrectomy, distal pancreatectomy, splenectomy and transverse colectomy for a GIST of the stomach that was categorized as high risk in 1993. Eight years later, the GIST relapsed on the gastrohepatic ligament, which led to left hepatectomy and cholecystectomy to excise the mass curatively. A year later, the tumor recurred in the liver, when imatinib (400 mg/d) was started as the initial treatment. Three years later, a follow-up computed tomography (CT) showed the progression of the hepatic metastasis, which resulted in an escalation in the imatinib dose to 600 mg/d. Unfortunately, two years later in April 2007, the hepatic mass grew so large that it invaded the transverse colon as evaluated by CT and colonoscopy (Figure 1). Consequently, we changed the therapy to oral sunitinib at 50 mg/d with a 4-wk-on and 2-wk-off regimen. He had no combined diseases but a history of smoking with two to thirty packs/year.
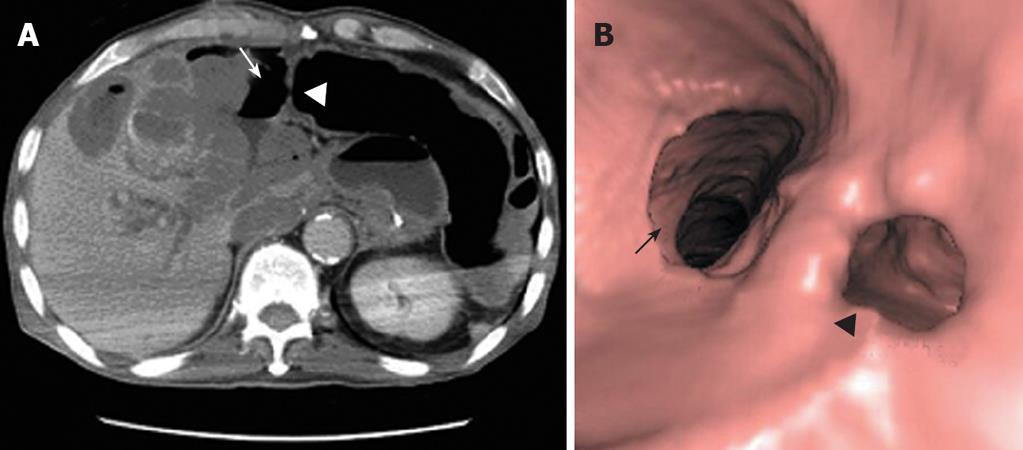
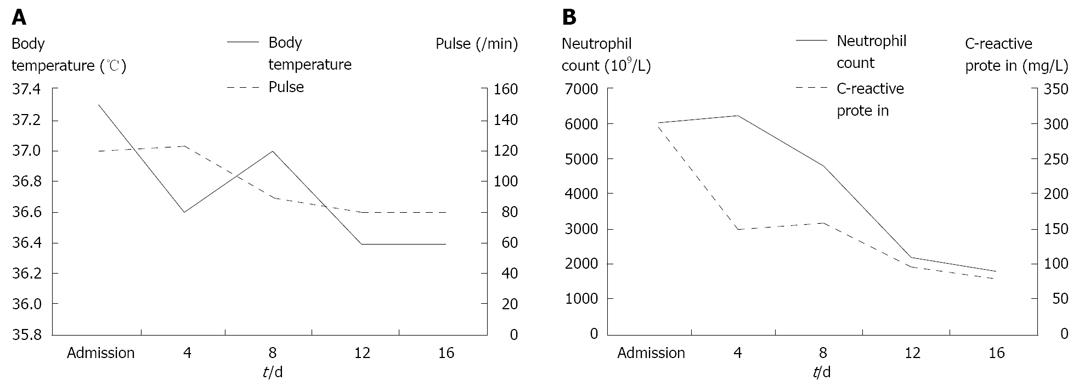
In June 2007, the patient was on the second day of the second cycle of sunitinib treatment and complained of diffuse abdominal pain and general prostration upon visiting our emergency room. The patient presented with symptoms and signs of localized peritonitis on the right side of the abdomen. The systolic and diastolic blood pressures were 90 mmHg and 60 mmHg, respectively, pulse rate was 120 per min and respiratory rate was 25 per min. The body temperature was 37.3°C. Laboratory studies were conducted immediately after the patient’s arrival at the emergency room. He had anemia (Hb 98 g/L) (normal range: 140-180) and thrombocytopenia (83 × 109/L) (normal range: 150-450), but a WBC count was normal (7.25 × 103/mm3) with 84% neutrophils. Other laboratory findings were presented as high serum levels of CRP (293.07 mg/L) (normal range: < 5), ALP (416 IU/L) (normal range: 96-254), slightly increased BUN (34 μmol/L) (normal range: 8-23) and creatinine (1.53 μmol/L) (normal range: 0.5-1.2), low level of sodium (127 mEq/L) (normal range: 135-148), potassium (2.8 mEq/L) (normal range: 3.5-5.1) and serum albumin (2.17 g/dL) (normal range: 3.8-5.3), and normal range of GOT (15 IU/L) (normal range: 13-36), GPT (8 IU/L) (normal range: 5-33). A CT scan showed necrosis of the recurrent hepatic mass and perforation of the invaded transverse colon, which led to intraperitoneal air and pus collection (Figure 2A). Three-dimensional reconstruction also revealed colonic perforation (Figure 2B). We could not perform the operation for a correction of colon perforation owing to the poor patient’s condition. With percutaneous drainage of the intraperitoneal abscess under guided ultrasonography, the patient’s diet was restricted to parenteral nutrition, intravenous fluid administration for correction of dehydration, and both cefoperazone (4 g/d, intravenous) and metronidazole (1500 mg/d, intravenous) were injected for 2 wk. After drainage procedure and conservative treatments, the patient’s condition was fortunately improved without operation and laboratory findings 2 wk after treatment were decreased serum CRP level (70.43 mg/L), normal range of platelets (413 × 109/L), BUN (11.3 μmol/L), creatinine (0.72 μmol/L), albumin (3.23 g/dL), sodium (139 mEq/L) and potassium (3.6 mEq/L) (Figure 3). The patient was discharged from the hospital after his condition improved. After completion of the second cycle, the disease was stable according to the response evaluation criteria in the solid tumor group (RECIST) with a 23% decrease in the diameter of the mass.
GIST is the most common mesenchymal neoplasm of the gastrointestinal tract. Although this tumor has been known as a neoplasm that is highly resistant to conventional chemotherapy, several recent findings of molecular abnormalities could provide a rationale for treatment with targeted therapies[2]. Recently, applications of several molecularly targeted therapies like imatinib and sunitinib have been proven sufficient for systemic treatment of inoperable or metastatic GIST.
Imatinib is a small molecule tyrosine kinase inhibitor with actions on various transduction molecules such as ABL, BCR-ABL, and KIT[4]. Some clinical trials have shown that imatinib therapy affects prolonged disease-free survival of advanced GIST patients. Sunitinib has recently received approval from the FDA for patients with GIST who are resistant to imatinib[3]. In one randomized controlled trial, sunitinib resulted in a significantly longer time of tumor progression than placebo in patients with GIST after failure of imatinib treatment[5]. Thus, the drug has become a new treatment strategy for these patients, since there is no proven second-line therapy for GIST. The molecular targets of sunitinib are RTKs, and this drug has also been known to have blocking effects on the activation of KIT or PDGFR, which has a similar mechanism of action to imatinib[1]. Moreover, sunitinib exerts an additional anti-angiogenic effect by inhibiting the activation of VEGFR[6]. Because of this effect, sunitinib has been considered beneficial for GIST patients who are resistant to imatinib or cannot tolerate it as a second line treatment[7,8]. A series of clinical studies on sunitinib reported that sunitinib can result in various adverse events, including hypertension, abnormalities of the thyroid and adrenal function, gastrointestinal symptoms such as constipation or diarrhea, and dermatologic abnormalities such as hand-foot syndrome[5,9]. However, bowel perforation associated with the use of sunitinib has not been reported to date. In this case, physical examination of localized tenderness and 3-D reconstructed CT scan confirmed the colon perforation. Then, the perforation was completely treated by percutaneous drainage without operation. This treatment strategy was possible because there was a small amount of colon content localized in a particular area of the peritoneum due to the adhesions from previous operations.
Several cases of colon perforation related to anti-cancer therapy have been reported[10,11], particularly with the VEGFR inhibitor bevacizumab (Avastin; Genentech, South San Francisco, CA), which shares one of the mechanisms of action of sunitinib. A randomized controlled clinical trial showed that bevacizumab, a humanized monoclonal antibody that binds to and neutralizes VEGF, led to gastrointestinal perforation in six of 393 patients (1.5%) who had metastatic colon cancer[10]. Furthermore, another case of colon perforation was documented after treatment with bevacizumab in a patient with non-small cell lung cancer[11]. In addition, the mechanism by which bevacizumab causes bowel perforation is attributed, at least in part, to the increased risk of arterial thrombosis that is observed in the use of drugs targeting VEGF[12,13]. Therefore, this drug is likely to generate rapid tumor degeneration, which in turn leads to colon perforation in patients with bowel metastasis or invasion, due to this inhibitory effect. In addition, the patient’s history of smoking might contribute to risk of colon perforation in our case. However, the mechanism of anti-angiogenic agents-induced colon perforation remains to be elucidated.
In conclusion, sunitinib, which is administered to treat GISTs resistant to imatinib, causes unexpected colon perforation and subsequent peritonitis. Hence, careful attention and appropriate clinical evaluation are required for patients presenting with gastrointestinal symptoms during sunitinib treatment.
Peer reviewers: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba; Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States
S- Editor Li DL L- Editor Wang XL E- Editor Zhang WB
| 1. | Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471-478. |
| 3. | Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107-113. |
| 4. | Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139-145. |
| 5. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. |
| 6. | Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327-337. |
| 7. | Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16-24. |
| 8. | Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, Cherrington JM, Mendel DB. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280-1289. |
| 9. | Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, Morgan JA, Dychter SS, Larsen PR, Demetri GD. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660-664. |
| 10. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. |
| 11. | Gray J, Murren J, Sharma A, Kelley S, Detterbeck F, Bepler G. Perforated viscus in a patient with non-small cell lung cancer receiving bevacizumab. J Thorac Oncol. 2007;2:571-573. |
| 12. | Ratner M. Genentech discloses safety concerns over Avastin. Nat Biotechnol. 2004;22:1198. |