Published online Oct 21, 2008. doi: 10.3748/wjg.14.6052
Revised: August 17, 2008
Accepted: August 24, 2008
Published online: October 21, 2008
AIM: To examine the expression of beta-catenin in colorectal cancer and look for association with other clinico-pathological parameters.
METHODS: Tumor samples from 163 cases of colorectal cancer (CRC) who had undergone primary colectomy between May, 1998 and November, 2002 with complete follow-up data for either 5 years or until death were recruited for a beta-catenin immunohistochemical study. The percentage of immunoreacted tumor cells was defined as overall staining density (OSD) and percentage of cells having nuclear localization was counted as nuclear staining density (NSD). Univariate exploration used log-rank test and multivariate survival analysis used Cox’s hazard regression model.
RESULTS: Beta-catenin immunoreactivity was detected in 161 samples (98.8%), of which 131 cases had nuclear staining. High OSD (≥ 75%), detected in 123 cases (75.5%), was significantly associated with earlier clinical staging (P < 0.01), lower nodal status (P = 0.02), non-metastatic status (P < 0.01) and better differentiation (P = 0.02). Multivariate analysis found that high OSD was independently associated with better survival [Cox’s hazard ratio 0.51, 95% confidence interval (CI) 0.31-0.83]. Although high NSD (≥ 75%) was correlated with high pre-operative serum CEA (P = 0.03), well differentiation (P < 0.01), and increased staining intensity (P < 0.01), the parameter was not significantly associated with survival.
CONCLUSION: Unlike previous reports, the study did not find a predictive value of nuclear beta-catenin in CRC. Instead, the overall expression of beta-catenin in CRC showed an association with better differentiation and earlier staging. Moreover, the parameter also independently predicted superior survival.
-
Citation: Wanitsuwan W, Kanngurn S, Boonpipattanapong T, Sangthong R, Sangkhathat S. Overall expression of
beta-catenin outperforms its nuclear accumulation in predicting outcomes of colorectal cancers. World J Gastroenterol 2008; 14(39): 6052-6059 - URL: https://www.wjgnet.com/1007-9327/full/v14/i39/6052.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6052
| Parameter | Cases | Overall staining density | P | |
| Low | High | |||
| Sex | ||||
| Male | 80 (49.3) | 18 (45.0) | 62 (50.8) | 0.52 |
| Female | 83 (50.6) | 22 (55.0) | 60 (49.2) | |
| Age (yr) | ||||
| < 65 | 87 (53.4) | 21 (52.5) | 66 (53.7) | 0.90 |
| > 65 | 76 (46.6) | 19 (47.5) | 57 (47.3) | |
| CEA | ||||
| < 5 ng/mL | 83 (50.9) | 25 (62.5) | 58 (47.1) | 0.09 |
| ≥ 5 ng/mL | 80 (49.1) | 15 (37.5) | 65 (52.9) | |
| AJCC staging | ||||
| Stage 1 | 20 (12.3) | 4 (10.0) | 16 (13.0) | 0.004 |
| Stage 2 | 61 (37.4) | 11 (27.5) | 50 (40.7) | |
| Stage 3 | 63 (38.7) | 14 (35.0) | 49 (39.8) | |
| Stage 4 | 19 (11.7) | 11 (27.5) | 8 (6.5) | |
| Tumor size | ||||
| T 0-1 | 25 (15.3) | 5 (12.5) | 20 (16.3) | 0.57 |
| T 2-3 | 138 (84.7) | 35 (87.5) | 103 (83.7) | |
| Nodal status | ||||
| N0 | 86 (52.8) | 17 (42.5) | 69 (56.1) | 0.02 |
| N1 | 39 (23.9) | 7 (17.5) | 32 (26.0) | |
| N2 | 38 (23.3) | 16 (40.0) | 22 (17.8) | |
| Metastatic status | ||||
| M0 | 144 (88.3) | 29 (72.5) | 115 (93.5) | < 0.001 |
| M1 | 19 (11.7) | 11 (27.5) | 8 (6.5) | |
| Differentiation | ||||
| Well | 83 (50.9) | 19 (47.5) | 64 (52.0) | 0.02 |
| Moderate | 60 (36.8) | 11 (27.5) | 49 (39.8) | |
| Poor | 20 (12.3) | 10 (25.0) | 10 (8.1) | |
| Staining localization | ||||
| Nuclear | 122 (50.3) | 19 (47.5) | 63 (51.2) | 0.68 |
| Non-nuclear | 31 (49.6) | 21 (52.5) | 60 (48.8) | |
| Staining intensity | ||||
| Weak to moderate | 54 (33.1) | 18 (45.0) | 34 (28.1) | 0.05 |
| Strong to very strong | 109 (66.9) | 22 (55.0) | 87 (71.9) | |
| NSD | ||||
| Low (< 75%) | 101 (47.2) | 33 (82.5) | 44 (35.8) | < 0.001 |
| High (≥ 75%) | 62 (52.8) | 7 (17.5) | 9 (64.2) | |
| Parameter | 5-yr DFS (%) | 5-yr OS (%) | Log-rank P |
| Overall | 54 | 57.7 | - |
| Sex | 0.77 | ||
| Male | 50.6 | 56.3 | |
| Female | 56.9 | 58.5 | |
| Age (yr) | 0.3 | ||
| < 65 | 55.7 | 59.7 | |
| ≥ 65 | 52.1 | 55.3 | |
| CEA | 0.09 | ||
| < 5 ng/mL | 59.7 | 65.1 | |
| ≥ 5 ng/mL | 48.2 | 50.0 | |
| AJCC staging | < 0.001 | ||
| Stage 1 | 68.6 | 75.0 | |
| Stage 2 | 81.2 | 86.9 | |
| Stage 3 | 39.2 | 41.3 | |
| Stage 4 | 0 | 0 | |
| Tumor size | 0.38 | ||
| T 0-1 | 61.9 | 72.0 | |
| T 2-3 | 52.4 | 55.1 | |
| Nodal status | < 0.001 | ||
| N0 | 73.1 | 79.1 | |
| N1 | 42.6 | 46.1 | |
| N2 | 21 | 21.1 | |
| Metastatic status | < 0.001 | ||
| M0 | 61.1 | 65.2 | |
| M1 | 0 | 0 | |
| Location | 0.03 | ||
| Colon | 61.6 | 65.8 | |
| Rectum | 46.1 | 49.4 | |
| Differentiation | 0.06 | ||
| Well | 61.9 | 62.0 | |
| Moderate | 49.4 | 49.5 | |
| Poor | 34.2 | 34.3 | |
| Staining localization | 0.09 | ||
| Nuclear | 69.2 | 73.3 | |
| Non-nuclear | 50.5 | 54.1 | |
| Staining intensity | 0.61 | ||
| Weak to moderate | 60.9 | 65.4 | |
| Strong to very strong | 50.7 | 54.1 | |
| OSD | < 0.001 | ||
| Low (< 75%) | 34.7 | 63.4 | |
| High (≥ 75%) | 60.5 | 40.0 | |
| NSD | 0.59 | ||
| Low (< 75%) | 51.1 | 55.8 | |
| High (≥ 75%) | 56.5 | 59.3 |
| Parameter | Intensity-adjusted 5-yr survival | |
| 5-yr DFS (%) | 5-yr OS (%) | |
| OSD | ||
| Low (< 75%) | 34.9 | 38.2 |
| High (≥ 75%) | 73.2 | 77.8 |
| NSD | ||
| Low (< 75%) | 55.3 | 58.4 |
| High (≥ 75%) | 68.7 | 75.7 |
| Parameter | Hazard ratio | 95% CI | P |
| Nodal status | 1.87 | 1.43-2.44 | < 0.001 |
| Metastatic status | 7.50 | 3.89-14.43 | < 0.001 |
| OSD | 0.51 | 0.31-0.83 | 0.007 |
According to the recent World Cancer Report[1], colorectal cancer (CRC) ranks as the third most common malignancy, following breast and lung cancers. The incidence of CRC in developing countries, including Thailand, has been increasing in recent years[2]. The increasing adoption of Western life style habits in these countries is believed to be responsible for the growing magnitude of the CRC problem. Although surgical therapy is the mainstay treatment for early stage CRC, there is an expanding role for multidisciplinary treatment. As adjuvant treatments are considered based on an individual patient risk, prognosticating factors are essential for risk stratification. Well accepted factors influencing outcome in CRC patients are tumor invasion, nodal status, lymphovascular invasion and serum carcinoembryonic antigen level[3,4]. Various histological parameters and molecular markers have been investigated and some have shown promising results in CRC, such as microvascular density[5] and microsatellite instability[6]. However, none of those biological factors have yet been integrated into the treatment algorithm.
Beta-catenin, a central molecule of the Wnt-signaling system, expresses in epithelial cells as two main forms; membrane localization and nuclear accumulation. Membrane localization can be detected in normal cells and tumor lineage, whereas nuclear beta-catenin is exclusively detected in immature cells and tumor cells[7]. Evidence of somatic mutations and nuclear accumulation of beta-catenin in various pediatric cancers signifies a role of the Wnt-signaling pathway in their tumorigenesis[8,9]. Accumulation of beta-catenin is a result of defects in its degradation process, which usually takes place in the cytoplasm by an interaction between beta-catenin and a complex of APC, AXIN and GSK-3-beta. Stabilized beta-catenin translocates into the nucleus where the protein acts as a transactivation factor and promotes tumor growth. In CRC, the molecular pathology involving members of the pathway has been elucidated[10]. Inactivation of APC, the tumor suppressor gene which regulates intracellular level of beta-catenin, is one of the earliest events observed in CRC development. Loss of APC function leads to pathologically increased cytoplasmic beta-catenin, which can be translocated into the nucleus or to the cell membranes[11]. At cell membranes, beta-catenin forms a complex with E-cadherin which plays a role as an adhesion molecule. Furthermore, Wnt-signaling may contribute to the process of colonic epithelial cells differentiation[12].
Recent studies have focused on the clinical meaning of nuclear beta-catenin accumulation in CRC and demonstrated its diagnostic as well as prognostic significance[13-19]. A high density of beta-catenin nuclear accumulation was associated with higher mortality in selected groups of CRC patients[14,18,19]. However, data from different series have been inconsistent. We conducted a study of beta-catenin immunohistochemistry in our clinical CRC series. With awareness that beta-catenin does not have only a growth promoting role, we did not limit our analysis to nuclear accumulation only, but also examined overall staining density against outcomes. The study failed to find evidence that nuclear beta-catenin accumulation could be a risk factor for unfavorable outcome in CRC. However, the study found a strong correlation between overall beta-catenin expression and better survival probability.
Archival tumor samples from 163 non-consecutive patients with histologically proven colonic adenocarcinoma who underwent primary resection at Songklanagarind Hospital, a tertiary teaching hospital in southern Thailand, between May, 1998 and November, 2002, were examined for this immunohistochemical study. CRC patients who were treated elsewhere, received a non-curative excision so that accurate pathological staging could not be determined, or were lost to follow-up after surgical treatment were not included in this study. All patients were evaluated for at least 5 years after surgery or until death. Survival status was evaluated by the institutional Tumor Registry Unit on December, 2007.
Before the surgical treatment, all patients had routine pre-operative investigations, including chest-X-rays, blood chemistry including serum albumin and carcinoembryonic antigen (CEA), and an evaluation for liver metastasis by ultrasonography and/or computerized tomography. Primary tumor staging followed the sixth edition of TNM staging system of the American Joint Committee on Cancer (AJCC)[20]. Adjuvant chemotherapy was given to stage III, colonic cancer patients, and adjuvant chemo-radiation was reserved for stages II and III rectal cancer cases. In stage IV CRC patients, chemoradiation therapy for advanced disease was considered for patients who were in status 0-2, according to the Eastern Cooperative Oncology Group. Follow-up visits were scheduled at 1-mo intervals during the first year after surgery, every 3-mo period during the second year, and every 6 mo, thereafter. Access to pathological samples and clinical records was approved by the institutional research ethics committee.
For analysis, age of the patients was stratified to less than 65 years and 65 years or more. Pre-operative serum CEA level was stratified at 5 ng/mL.
Hematoxylin and eosin stained slides were re-examined by a gastrointestinal pathologist and selected for the beta-catenin immunohistochemical study. From formalin-fixed paraffin embedded tissue, sections of 3 micrometer thickness were cut, deparaffinized, and rehydrated. Beta-catenin monoclonal antibody (1:500, Abcam Plc., UK) was used as the primary antibody. The staining protocol followed the manufacturers’ instructions of Dako EnVision + System (Dako). Briefly, antigen retrieval was performed in a microwave oven using Tris-EDTA buffer. Endogenous peroxidase activity was blocked with 0.03% hydrogen peroxide containing sodium azide. Slides were incubated with non-immune serum for 30 minutes and were then incubated with the primary antibody for 120 minutes in a moist chamber, followed by a 30 minute incubation with peroxidase labeled polymer conjugated to goat anti-mouse immunoglobulins. Color was then developed by the liquid 3,3'-diaminobenzidine chromogen solution. Light counterstaining was done with hematoxylin. All immunohistological staining in this study was performed by one technician.
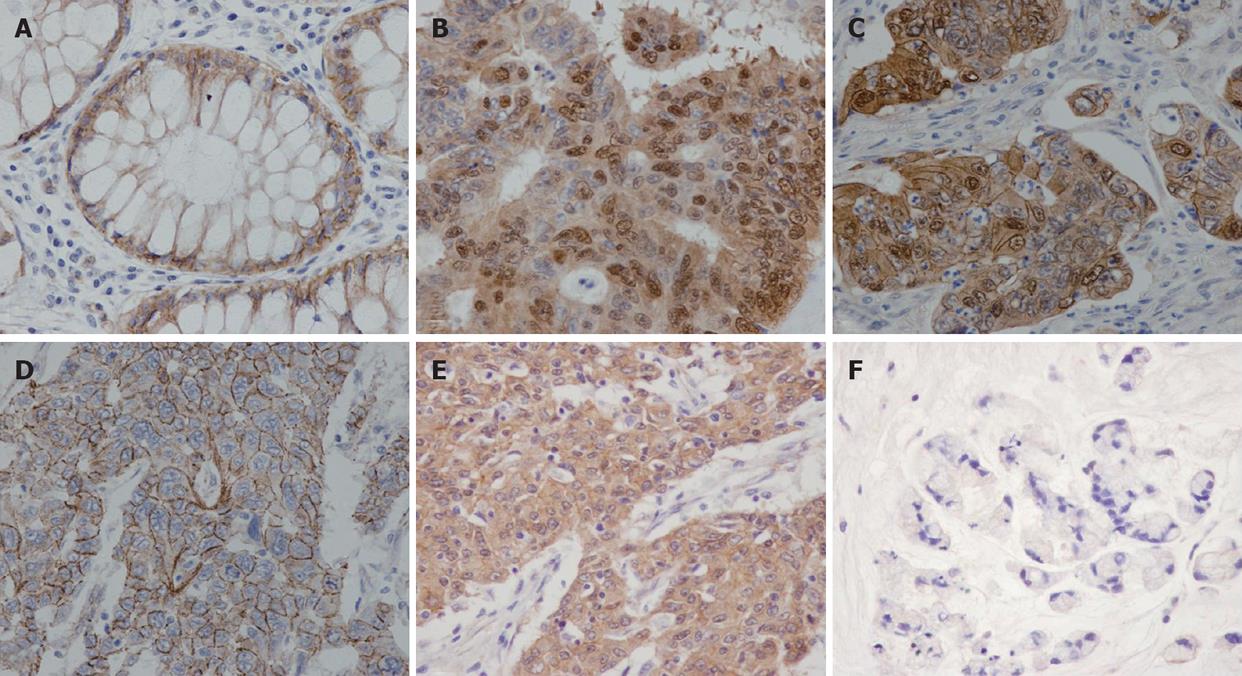
Staining intensity of beta-catenin was graded as weak, moderate, strong and very strong intensity. Intracellular localization patterns of beta-catenin were categorized according to the dominant pattern as nuclear staining, nuclear together with membrane staining, cytoplasm staining and membrane staining.
Beta-catenin nuclear staining density (NSD) was defined as the number of tumor cells with nuclear staining per 100 cells examined. Overall staining density (OSD) meant number of beta-catenin immunoreacted cell per 100 tumor cells examined. Tumors were defined as having high NSD when NSD was 75% or more, and OSD at 75% or more indicated high OSD. The pathologists responsible for histological slide examination were blinded to the clinical outcomes before data analysis.
Death from cancer was assigned as failure in the overall survival analysis. Local recurrence and distant metastasis were counted as failure in disease-free survival, which were analyzed only for cases in AJCC stage I-III. Univariate analysis used Chi-square test to evaluate any association between parameters and Log rank test for survival analysis. Multivariate survival analysis used a multiple Cox’s regression model. Parameters with P-value less than 0.3 from the Log rank test were included for analysis. Those with a P-value ≥ 0.05 in Cox’s regression were excluded until every parameter in the model was independently associated with survival. A P-value of less than 0.05 was considered statistically significant.
Calculations used the R-program (Foundation for Statistical Computing, Vienna, Austria).
The clinical characteristics and pathological descriptions of the 163 patients are shown in Table 1. The mean age of the patients was 61.6 years (range 20-88 years). Median pre-operative serum CEA was 4 ng/mL (range 0.5-1215 ng/mL). Eleven of the primary tumors (6.7%) were at the cecum, 13 (8.0%) at the ascending colon, 5 (3.0%) at the hepatic flexure colon, 11 (6.7%) at the transverse colon, 2 (1.2%) at the splenic flexure colon, 3 (1.8%) at the descending colon, 37 (22.7%) at the sigmoid colon and 81 (49.7%) at the rectum.
Beta-catenin immunoreactivity was detected in 161 cases (98.8%). Among these, 131 cases (80.3% of all cases) were positive for nuclear beta-catenin staining while the remaining cases showed limited immunoreactivity within the cell membranes and/or cytoplasm. Among cases with positive nuclear beta-catenin, 40 (30.5%) were also positive for membrane immunoreactivity. Concerning staining intensity, 74 cases (45.4%) displayed very strong immunoreactivity, 35 (21.5%) showed strong intensity, 27 (16.6%) had moderate intensity, and 25 (15.5%) cases exhibited weak staining (Figure 1). Interestingly, nuclear beta-catenin was detected in a significantly higher proportion in cases with strong or very strong intensity (99.1%), compared to cases with moderate or weak immunoreactivity (44.23%). Cases with positive nuclear staining had a higher incidence of lymph node metastasis (50.3%) when compared to cases with only membranes or cytoplasm staining (33.3%), however, the difference was not statistically significant (Chi-square P-value = 0.09).
Overall staining density ranged from 5% to 100% with an average value of 86%. One hundred and twenty-three cases (75.5%) were rated as high OSD. On univariate analysis, high OSD was significantly associated with earlier AJCC staging, lower nodal status, non-metastatic status and better differentiation status (Table 1).
Among the 131 cases in which the nuclear staining appeared positive, nuclear accumulation density ranged from 1% to 99 % with an average value of 65.6%. Eighty-six cases (52.8%) had nuclear accumulation density at 75% or more and were counted as high NSD. High NSD had significantly positive correlation with pre-operative serum CEA (P = 0.03), well differentiation (P < 0.001), and staining intensity (P < 0.001). In addition, cases with NSD at 25% or more had significantly higher incidence of nodal metastasis at the operation (P = 0.01).
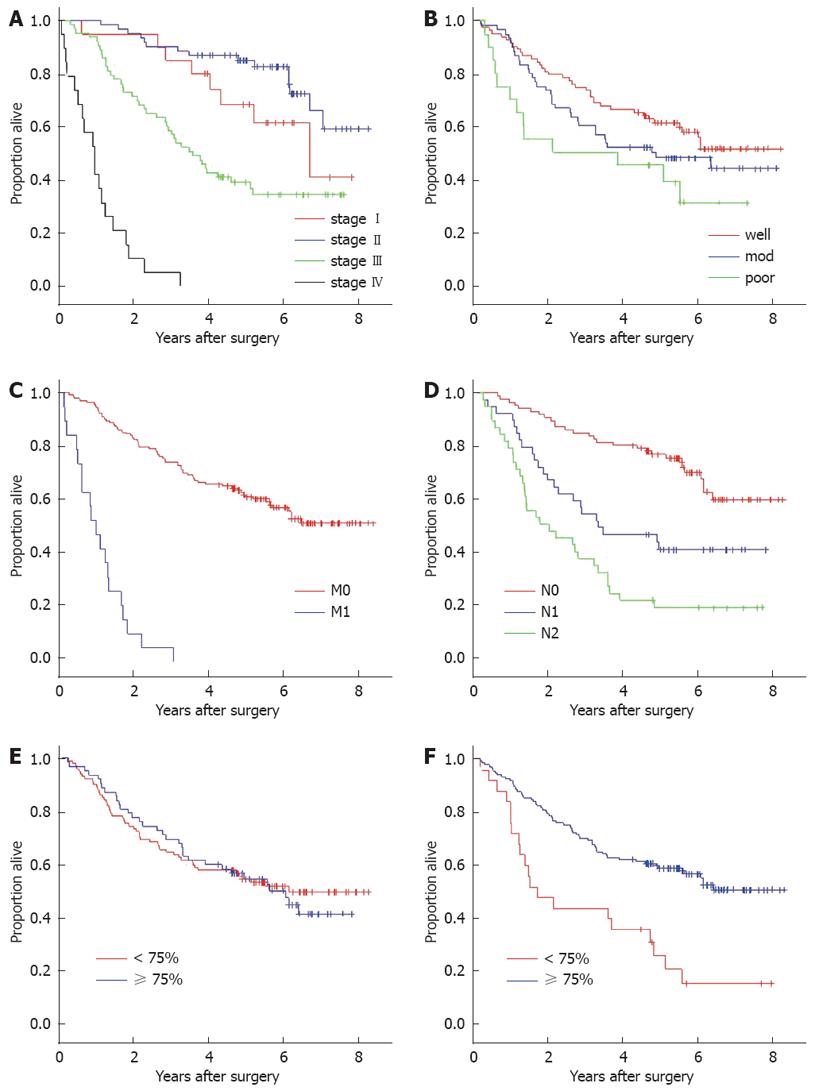
There were no missing data in this study. The mean follow-up period was 56.2 mo. Overall survival and disease-free survival at 5-years were 57.7% and 54.0%, respectively. Univariate analysis found that AJCC staging, nodal status, metastatic status and tumor location were among the clinical parameters that had significant association with both overall survival and disease-free survival. Tumor differentiation was correlated with disease-free survival and the overall survival only at borderline significance (Log rank P-values 0.05 and 0.06, respectively) (Table 2). CEA at 5 ng/mL or more predicted significantly poorer outcome only in the AJCC stage I subgroup (Log rank P-value 0.045).
Five-year overall survival in patients with high NSD (59.3%) was not different from cases with low density (55.8%) when compared by Log-rank analysis (P = 0.59). On the other hand, OSD showed a significant influence on both overall (P < 0.001) and disease-free survival probability (P < 0.001) (Figure 2). Two and five-year overall survival rates in cases with high OSD (84.6% and 60.5%, respectively) were obviously higher than low OSD (50.0% and 34.7%, respectively). Adjusting for the staining intensity resulted in a greater difference of 5-year overall survival probability between the high OSD (77.8%) and low OSD group (38.2%) (Table 3).
With Cox’s hazard multivariate analysis, the final model demonstrated that perioperative parameters that were independently associated with survival probability in colorectal cancer were nodal status, metastatic status, and OSD (Table 4). When subgroup analysis was performed for cases without metastasis at the time of diagnosis, nodal status (hazard ratio 2.0, P < 0.001), OSD (hazard ratio 0.36, P < 0.001), serum CEA at 5 ng/mL or more (hazard ratio 1.8, P < 0.001) and differentiation status (hazard ratio 1.4, P < 0.02) were significantly associated with disease-free survival. Within the same non-metastatic subgroup, factors that significantly fit the final regression model analyzing overall survival were nodal status (hazard ratio 2.3, P < 0.001), OSD (hazard ratio 0.4, P < 0.001), and age more than 65 years (hazard ratio 1.6, P < 0.04).
Excluding OSD from the model, the Cox’s hazard analysis showed that high NSD had a hazard ratio of 2.04 [95% confidence interval (CI) 1.0-4.2], compared with NSD less than 50, when adjusted for tumor stage, differentiation, nodal and metastatic status.
An outcome prognosticating factor is an essential component for risk categorization in utilizing the risk-based therapy concept. Defining an individual at-risk of unfavorable prognosis helps in selecting patients who are most likely to benefit from intensive adjuvant treatment. Earlier successful examples of risk-based therapy in biological factors have been integrated into the management scheme are neuroblastoma[21], breast cancer[22] and gastrointestinal stromal tumors[23]. In CRC, although various biological outcome predictors have been discovered, those parameters have not yet been adopted into a standard treatment protocol of CRC.
The Wnt-signaling pathway plays several roles in humans; a physiological role in normal development and a pathological role in tumorigenesis[7]. A common denominator of the pathway activation is the intracellular amount and localization of beta-catenin, the central molecule. The carcinogenic process of CRC could be linked to the Wnt-signaling cascade through the loss of APC function, which has been attributed to up to 60% of sporadic tumors[24]. Because APC is essential for clearance of excessive intracellular beta-catenin, accumulation of beta-catenin occurs as a result of APC down-regulation. Positive nuclear accumulation has been reported between 21%-100% in sporadic CRC[15-17,19], depending on the characteristics of the patients in the series, the staining protocol and their histological parameter. In our study, tumor cells with nuclear staining was detected in at least 80% of the cases, however, only 66% of these cases had high NSD. In a series of 136 patients with CRC, Cheah and colleagues identified a negative survival influence of nuclear beta-catenin[15]. In their study, the nuclear beta-catenin was covariate with tumor stage; however, it could be a strong prognosticator when combined with another factor p27b12. Two publications by Wong and colleagues reported that progression of nuclear beta-catenin immunohistochemistry scores was correlated with advances in the malignant potential of colonic neoplasms[13,14]. The studies also showed survival differences between cases with high and low expression density of nuclear beta-catenin. Outcome prognosticating ability of non-membranous beta-catenin was also supported by another two other separate works from Europe[16,17]. Lines of epidemiological evidence suggested that nuclear accumulation of beta-catenin could be employed as a factor identifying cases at risk of treatment failure. However, consensus histological criteria had not been established at the time of there studies and most of them focused only on nuclear localization.
Our study aimed to evaluate the roles of beta-catenin in our clinical series of CRC patients in whom the therapeutic process was homogenously performed by the same multidisciplinary team and the follow-up period was long enough to give reliable data. Apart from nodal status, pre-operative CEA and chronological age predicted disease-free survival and overall survival non-metastatic CRC in the series. Although NSD showed no significant survival function, hazard analysis demonstrated that the factor was associated with mortality risk when other factors were adjusted. Moreover, high NSD was linked to lymph node metastasis and high serum CEA, a finding consistent with previous reports which found that nuclear-accumulated beta-catenin predicted an unfavorable outcome. However, in our series, the influence of nuclear beta-catenin was not independent from other major prognosticators. On the other hand, when we analyzed the overall expression of beta-catenin in term of OSD, it was found that the factor was strongly associated with survival advantage. This conflicting data could not be simply explained by using the mainstream theory of the Wnt-signaling cascade. A positive correlation between NSD, OSD and staining intensity was one of the interesting findings in our study. If NSD is regarded as a localization parameter of beta-catenin and OSD represents an overall expression level of the protein, it appears that the two parameters are co-variant and OSD has much stronger influence on survival.
Besides the growth promoting function, alternative roles of beta-catenin should not be overlooked. Beta-catenin performs necessary physiologic tasks in cellular differentiation and cell-to-cell adhesion[25]. Our study detected a significant correlation between beta-catenin expression and tumor differentiation. Poorly differentiated tumors harbored a significantly smaller proportion of high NSD and OSD. This association was also shown in previous series, although the survival correlation appeared in an opposite direction[15]. A recent study from our group demonstrated that beta-catenin promotes differentiation in another malignancy, neuroblastoma[26]. If these things are taken together, it might be hypothesized that beta-catenin plays a role in maintaining a good differentiation status in cancer cells. Furthermore, the physiologic function of beta-catenin at the cell membranes is related to cell-to-cell adhesion, which possibly prevents metastasis. A study by Lugli[18] and colleagues found that membranous beta-catenin co-localized with E-cadherin and loss of membranous or cytoplasmic beta-catenin characterized a higher stage disease. In our series, loss of OSD was evident in advanced stage CRC, and there was also a significantly higher OSD proportion in early nodal status. The physiologic functions of beta-catenin in tumor cell differentiation and adhesion may explain our findings. However, to conclude that beta-catenin expression provides a protective role in CRC, further functional genetics research needs to be performed.
In summary, expression and localization of beta-catenin immunohistochemistry in a series of CRC were analyzed. The study did not find a prognosticating role of the nuclear localization; however, overall expression of beta-catenin was found as a strong and independent predictor of favorable outcome.
The authors examined the expression of beta-catenin in colorectal cancer and looked for association with other clinicopathological parameters, and outcomes.
Beta-catenin immunohistochemistry was performed in 163 cases of colorectal cancer in whom the outcome data was clearly available.
The study found certain data that was not in-line with previous reports. Instead of nuclear beta-catenin that was associated with survival, the overall staining density of this protein showed strong and independent correlation with overall survival and disease-free survival. Moreover, the parameter (overall staining density) also had positive association with tumor differentiation.
The data suggested that beta-catenin may have an alternative role in colorectal cancer that was associated with differentiation of tumor cells.
Beta-catenin nuclear staining density (NSD) was defined as the number of tumor cells with nuclear staining per 100 cells examined. Overall staining density (OSD) meant number of beta-catenin immunoreacted cell per 100 tumor cells examined. Tumors were defined as having high NSD when NSD was 75% or more, and OSD at 75% or more indicated high OSD.
This is a well analyzed paper and provides a new insight into the importance of beta catenin staining as a prognostic marker in colorectal cancer.
Peer reviewer: Finlay A Macrae, MD, Professor, Royal Melbourne Hospital, Po Box 2010, Victoria 3050, Australia
S- Editor Xiao LL L- Editor Rippe RA E- Editor Zhang WB
| 1. | Stewart BW, Kleihues P. World cancer report. Lyon: IARC Press 2003; . |
| 2. | Khuhaprema T, Srivatanakul P. Colon and rectum cancer in Thailand: an overview. Jpn J Clin Oncol. 2008;38:237-243. |
| 3. | Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757. |
| 4. | Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40:592-595. |
| 5. | Acikalin MF, Oner U, Topcu I, Yasar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37:162-169. |
| 6. | Lurje G, Zhang W, Lenz HJ. Molecular prognostic markers in locally advanced colon cancer. Clin Colorectal Cancer. 2007;6:683-690. |
| 8. | Koesters R, von Knebel Doeberitz M. The Wnt signaling pathway in solid childhood tumors. Cancer Lett. 2003;198:123-138. |
| 9. | Sangkhathat S, Kusafuka T, Miao J, Yoneda A, Nara K, Yamamoto S, Kaneda Y, Fukuzawa M. In vitro RNA interference against beta-catenin inhibits the proliferation of pediatric hepatic tumors. Int J Oncol. 2006;28:715-722. |
| 10. | Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854-865. |
| 11. | Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477-487. |
| 12. | de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471-491. |
| 13. | Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401-1408. |
| 14. | Wong SC, Lo ES, Chan AK, Lee KC, Hsiao WL. Nuclear beta catenin as a potential prognostic and diagnostic marker in patients with colorectal cancer from Hong Kong. Mol Pathol. 2003;56:347-352. |
| 15. | Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer. 2002;95:2479-2486. |
| 16. | Bondi J, Bukholm G, Nesland JM, Bukholm IR. Expression of non-membranous beta-catenin and gamma-catenin, c-Myc and cyclin D1 in relation to patient outcome in human colon adenocarcinomas. APMIS. 2004;112:49-56. |
| 17. | Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790-2796. |
| 18. | Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, Jass JR. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. 2007;50:453-464. |
| 19. | Martensson A, Oberg A, Jung A, Cederquist K, Stenling R, Palmqvist R. Beta-catenin expression in relation to genetic instability and prognosis in colorectal cancer. Oncol Rep. 2007;17:447-452. |
| 20. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag 2002; . |
| 22. | Acharya CR, Hsu DS, Anders CK, Anguiano A, Salter KH, Walters KS, Redman RC, Tuchman SA, Moylan CA, Mukherjee S. Gene expression signatures, clinicopathological features, and individualized therapy in breast cancer. JAMA. 2008;299:1574-1587. |
| 23. | Takahashi T, Nakajima K, Nishitani A, Souma Y, Hirota S, Sawa Y, Nishida T. An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol. 2007;12:369-374. |
| 24. | Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531-7537. |
| 25. | Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820-828. |