Published online Oct 21, 2008. doi: 10.3748/wjg.14.6018
Revised: September 23, 2008
Accepted: September 30, 2008
Published online: October 21, 2008
AIM: To analyze, retrospectively in a population-based study, the management and survival of patients with recurrent rectal cancer initially treated with a macroscopically radical resection obtained with total mesorectal excision (TME).
METHODS: All rectal carcinomas diagnosed during 1998 to 2000 and initially treated with a macroscopically radical resection (632 patients) were selected from the Amsterdam Cancer Registry. For patients with recurrent disease, information on treatment of the recurrence was collected from the medical records.
RESULTS: Local recurrence with or without clinically apparent distant dissemination occurred in 62 patients (10%). Thirty-two patients had an isolated local recurrence. Ten of these 32 patients (31%) underwent radical re-resection and experienced the highest survival (three quarters survived for at least 3 years). Eight patients (25%) underwent non-radical surgery (median survival 24 mo), seven patients (22%) were treated with radio- and/or chemotherapy without surgery (median survival 15 mo) and seven patients (22%) only received best supportive care (median survival 5 mo). Distant dissemination occurred in 124 patients (20%) of whom 30 patients also had a local recurrence. The majority (54%) of these patients were treated with radio- and/or chemotherapy without surgery (median survival 15 mo). Twenty-seven percent of these patients only received best supportive care (median survival 6 mo), while 16% underwent surgery for their recurrence. Survival was best in the latter group (median survival 32 mo).
CONCLUSION: Although treatment options and survival are limited in case of recurrent rectal cancer after radical local resection obtained with TME, patients can benefit from additional treatment, especially if a radical resection is feasible.
- Citation: Bakx R, Visser O, Josso J, Meijer S, Slors JFM, Lanschot JJBV. Management of recurrent rectal cancer: A population based study in greater Amsterdam. World J Gastroenterol 2008; 14(39): 6018-6023
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/6018.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6018
| Stage of disease | Number of cases (% of total) | Radiotherapy, number of patients (%) | ||
| No RT | Postoperative RT | Preoperative RT | ||
| I | 209 (33) | 115 (55) | 1 (0) | 93 (45) |
| IIA | 180 (28) | 72 (40) | 26 (14) | 82 (46) |
| IIB | 20 (3) | 2 (10) | 6 (30) | 12 (60) |
| IIIA | 32 (5) | 8 (25) | 11 (34) | 13 (41) |
| IIIB | 113 (18) | 26 (23) | 32 (28) | 55 (49) |
| IIIC | 72 (11) | 13 (18) | 23 (32) | 36 (50) |
| Unknown | 6 (1) | 2 (33) | - | 4 (67) |
| Total | 632 | 238 (38) | 99 (16) | 295 (47) |
| Secondary treatment | Stage and treatment with radiotherapy at initial diagnosis | |||||||||
| Stage I | Stage II | Stage III | Total n (%) | |||||||
| No RT | Post RT | Pre RT | No RT | Post RT | Pre RT | No RT | Post RT | Pre RT | ||
| Radical surgery1 (± radiotherapy and/or chemotherapy) | 3 | - | 1 | 3 | - | 1 | 1 | - | 1 | 10 (3) |
| Non-radical surgery1 (± radiotherapy and/or chemotherapy) | - | - | - | 2 | 1 | 2 | - | 3 | - | 8 (2) |
| Radiotherapy and/orchemotherapy without surgery | 2 | - | - | 1 | 1 | - | 1 | 2 | - | 7 (2) |
| Best supportive care | 1 | - | - | - | 1 | 3 | 1 | - | 1 | 7 (2) |
| Total | 6 | - | 1 | 6 | 3 | 6 | 3 | 5 | 2 | 32 |
| Parameter | Number of cases | HR (95% CI) |
| Sex | ||
| Male (reference) vs female | 33/29 | 1.9 (0.9-3.7) |
| Radiotherapy at initial treatment | ||
| No radiotherapy | 23 | 1.0 |
| Preoperative radiotherapy | 23 | 1.2 (0.6-2.5) |
| Postoperative radiotherapy | 16 | 0.9 (0.4-1.8) |
| Distant dissemination at time of local recurrence | ||
| Absent (reference) vs present | 32/30 | 0.8 (0.4-1.6) |
| Surgical treatment of locally recurrent disease | ||
| No surgery | 42 | 1.0 |
| Radical surgery | 10 | 0.1 (0.0-0.3) |
| Non-radical surgery | 10 | 0.5 (0.2-1.3) |
Colorectal cancer is the second most common cancer in the Western world and approximately one third of these tumours are located in the rectum or rectosigmoid[1]. Annually, over 3000 patients are registered with a newly diagnosed rectal or rectosigmoid carcinoma in the Netherlands[2,3]. In these patients, locally recurrent disease is a major concern and is often accompanied with intractable pain and severely disabling complications which are difficult to treat[4-6]. It has a tremendous impact on quality of life[7] and frequently induces an awful last period of a patient's life. Therefore, the focus in rectal cancer research has been on the prevention of locally recurrent disease, which resulted in the introduction of preoperative radiotherapy and total mesorectal excision (TME)[8-12].
There are many reports on the treatment of recurrent rectal cancer[4,6,13-16]. However, these reports present mainly results from randomised clinical trials or specialised institutes, which are known to be biased[17]. There are only a few population-based reports on the treatment of locally recurrent rectal cancer disease[13,18], although they are probably the best reflection of daily practice.
In 1996, TME was introduced in Greater Amsterdam, the region of the Comprehensive Cancer Centre Amsterdam (CCCA). Its introduction was facilitated by the CCCA. Surgeons were supervised by teacher-surgeons in order to qualify as TME-surgeon and a documentation project was started to investigate the influence of TME-surgery on the incidence of local recurrences and survival[19]. From 1998 on, all patients in Greater Amsterdam are treated with TME in case of rectal resection.
The aim of the present study was to analyze, retrospectively in a population-based setting, the management and survival of patients with recurrent rectal cancer, initially treated with macroscopically radical local resection obtained with TME.
All primary rectal carcinomas (rectosigmoid excluded) diagnosed in patients with residence in Greater Amsterdam, the region of the CCCA, between January 1, 1998 and December 31, 2000, and who underwent a macroscopically radical resection obtained with TME in the absence of distant dissemination, were selected from the Amsterdam Cancer Registry of the CCCA. The Amsterdam Cancer Registry is a regional, population-based cancer registry with complete regional coverage. Non-epithelial cancers, carcinoids and cases with preceding invasive cancers were excluded. The population of the region amounted to 2.8 million inhabitants on December 31, 2000, approximately 17% of the total population of the Netherlands.
The information for the cancer registry is routinely extracted from detailed hospital and outpatient clinic records by registration clerks. Apart from demographic data, data are collected on morphological classification, stage of the tumour and primary treatment of the patients. The TNM system for classification of malignant tumours is prospectively registered to classify all rectal carcinomas. Stage grouping in this study was performed according to the 6th edition of the TNM-classification[20], based on the available information after surgery (pTNM).
Of the selected cases, a supplementary data set was extracted from the medical records. This data set included the occurrence and the date of local recurrence or distant dissemination. Local recurrence was defined as cancer recurrence within the lower pelvis. Additional treatment of recurrence, the presence of microscopic or macroscopic residual disease after salvage surgery for recurrent disease, the date of salvage surgery and the cause of death were also collected. Cases were generally followed for five, but at least three years after the date of initial surgery.
The vital status was updated by active follow-up in the hospitals, by linking files with deceased persons to the cancer registry and by linkage to the electronic death registry of the Central Office for Genealogy in September, 2003 and February, 2005, as described earlier[21]. Completeness of follow-up of the vital status is estimated to be over 99.5%.
P < 0.05 was considered statistically significant. All statistical analyses were performed using a two-sided 5% level of significance.
Survival probabilities were estimated using the Kaplan-Meier method[22]. Multivariate analyses using the Cox proportional-hazard method were performed to calculate the hazard ratio (HR) for death after recurrent disease[23]. Cox regression and Kaplan-Meier survival curves were calculated with STATA (Stata Corporation, College Station, TX, USA).
A total of 632 patients diagnosed with primary rectal carcinoma in the absence of clinically manifest distant dissemination between 1998 and 2000 underwent a macroscopically radical local resection obtained with TME. Characteristics of the initial treatment of the primary tumour in these patients are given in Table 1. Local recurrence within five years after diagnosis occurred in 62 patients (10%), including 30 cases with distant dissemination (6%). Of these 30 patients, 24 patients had synchronous local and distant recurrence, while 6 patients developed distant dissemination after the local recurrence.
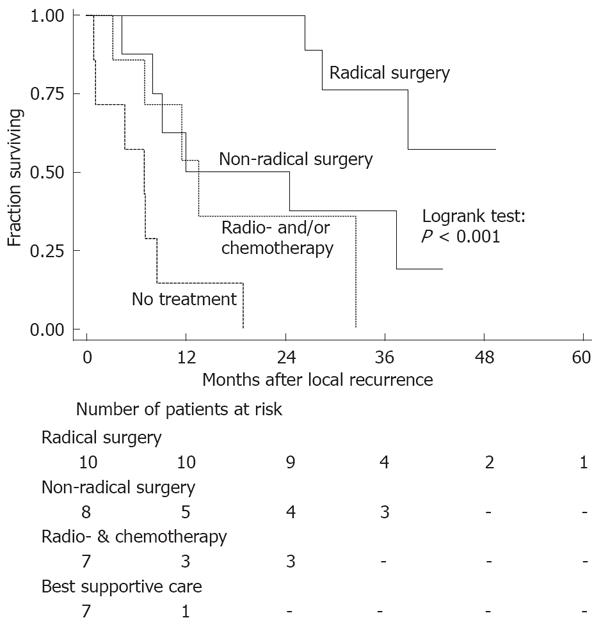
There were 32 out of 62 patients (52%) without signs of distant dissemination at the time of diagnosis of recurrent disease. Median survival after recurrence in the absence of distant dissemination was 25 mo. Ten of these 32 patients underwent a microscopically radical resection of their recurrence (Table 2). As is depicted in Figure 1, radical surgery resulted in a significantly better survival than non-radical surgery, radio- and/or chemotherapy without surgery or best supportive care (log-rank test radical surgery vs other treatments: P < 0.001). About three quarters of the patients who underwent a radical resection survived for at least three years. Median survival after non-radical surgery (8 patients) was 24 mo, 7 mo after radio- and/or chemotherapy without surgery (7 patients) and was 5 mo in case of best supportive care only (7 patients).
In 30 patients (48%), distant dissemination was present at the time of diagnosis of local recurrent disease. Median survival after local recurrence in the presence of distant dissemination was 10 mo. None of these patients underwent curative surgery, two patients underwent non-radical surgical resection, 14 patients were treated with radio- and/or chemotherapy without surgery (median survival 14 mo) and 14 patients received best supportive care only (median survival 9 mo).
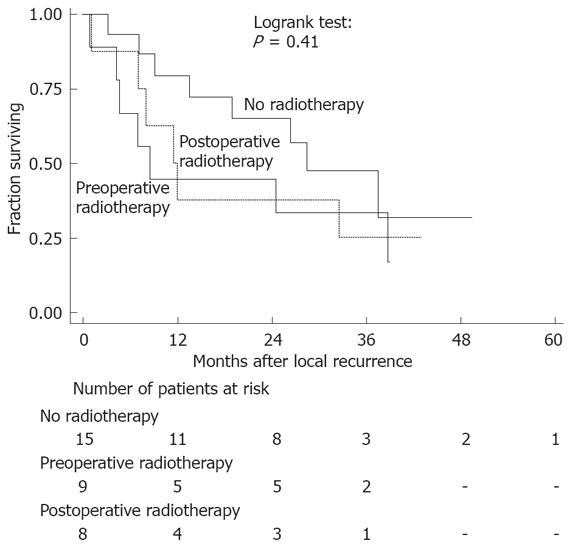
Several factors were analysed to identify prognostic factors for improved survival after local recurrence. The results of the multivariate analysis are shown in Table 3. Surgery for recurrent disease (radical and non-radical) was a prognostic factor for improved survival, while radiotherapy applied during the initial treatment did not influence survival after local recurrence (Figure 2).
Distant dissemination within five years after diagnosis occurred in 124 patients (20%). The majority of patients (54%) with distant dissemination were treated with radio- and/or chemotherapy (Table 4). The median survival after distant dissemination was 15 mo.
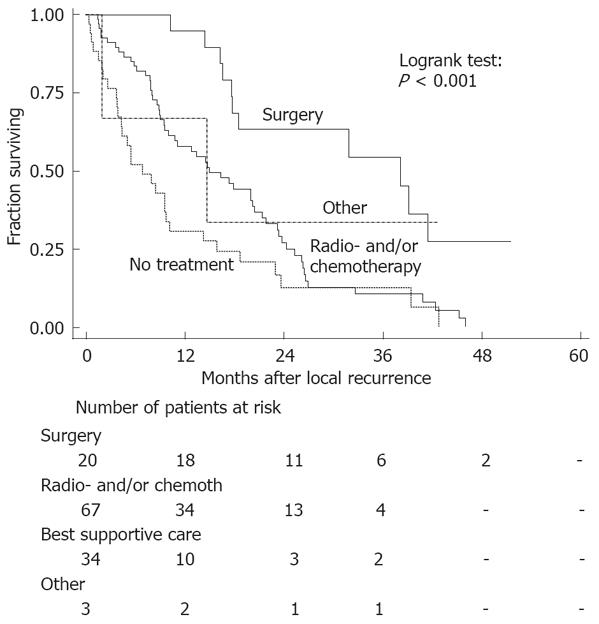
Twenty patients (16%) underwent surgery for their recurrence, including liver resections in eight patients, lung resections in five patients, and other surgical procedures in seven patients. Median survival after surgery was 32 mo, while median survival after radiotherapy and/or chemotherapy without surgery was 15 mo and 6 mo if best supportive care was applied (Figure 3). Patients with distant dissemination who were treated surgically experienced the highest survival (log-rank test surgery vs other treatments: P < 0.001).
This is the first population-based study concerning recurrent rectal cancer treatment after the introduction of TME. All patients in this study were initially diagnosed between 1998 and 2000 in Greater Amsterdam and treated by macroscopically radical local resection obtained with TME. A local recurrence occurred in 62 of the 632 patients (10%), while distant dissemination was found in 124 patients (20%).
Of the 32 patients with an isolated local recurrence, 31% were treated by a radical resection. These patients experienced a significantly better survival compared to patients who underwent a non-radical resection for their recurrence. As has been shown previously, radical resection of locally recurrent disease can achieve long-term survival[4,13-15,24] and should, therefore, be aimed at, even if extended resection (e.g. abdominosacral resection or exenteration)[16,25,26] or flap-reconstruction[27] is required.
Survival in patients treated with non-radical surgery and patients treated with radiotherapy and/or chemotherapy without surgery was comparable, but was significantly worse in patients not treated with surgery, radiotherapy or chemotherapy. Although no information concerning the extent of recurrent disease was available in this study, treatment has probably been more aggressive in case of limited disease and, therefore, selection bias may have played an important role in the outcome of the various treatment modalities.
The median survival after distant dissemination was 15 mo for patients diagnosed between 1998 to 2000. In a previous study, we have described that patients diagnosed in 1988 between 1991 in Greater Amsterdam only survived 9 mo after distant dissemination (log-rank test: P = 0.004)[19]. The majority of patients diagnosed between 1998-2000 (54%) with distant dissemination were treated with radiotherapy and/or chemotherapy without surgery, while 16% were treated with surgical resection and 27% received only best supportive care. Survival was significantly better in the group of patients treated with surgery compared to other groups. This is probably due to the limited spread of disease in these patients (selection bias). As no treatment data were available for the patients diagnosed between 1988 to 1991 in Greater Amsterdam, it is unclear which treatment modality has contributed to the increase in the median survival.
In this population-based study, treatment options and survival were limited in patients with recurrent rectal cancer after macroscopically radical local resection obtained with TME. Approximately one third of the patients only received best supportive care with a subsequent poor survival. On the other hand, in one third of the patients with an isolated local recurrence, radical resection was feasible with a favourable survival. We conclude that a locally recurrent rectal cancer without distant dissemination does not automatically lead to a hopeless situation[28]. However, survival after local recurrence in combination with distant dissemination remains extremely poor.
Colorectal cancer is a common cancer in the Western world and rectal cancer has been subject to significant treatment changes over the last decade. Despite this change, recurrent rectal cancer treatment remains a frustrating and ongoing process.
In the recent decade, the main changes in rectal cancer treatment have been the application of preoperative radiotherapy and the introduction of total mesorectal excision (TME) which led to a significant decrease in local recurrence percentages. In the current article, the influence the introduction of TME on recurrent rectal cancer treatment is evaluated in a population based cohort.
The current results show that recurrent rectal cancer treatment is in some cases worthwhile, especially if a radical resection is feasible. However, avoiding a local recurrence remains an important aspect in future rectal cancer treatment.
TME is a surgical technique initiated by B. Heald during which the entire mesorectum is excised using the so-called “holy” plane. This provides the opportunity to remove the entire rectum with possibly infiltrated lymph nodes.
This is an interesting study which was well organized. It demonstrated that although treatment options and survival are limited in case of recurrent rectal cancer after radical local resection obtained with TME, patients can benefit from additional treatment, especially if a radical resection is feasible.
Peer reviewer: Bernardino Rampone, PhD, Department of General Surgery and Surgical Oncology, University of Siena, viale Bracci, Siena 53100, Italy
S- Editor Li DL L- Editor Rippe RA E- Editor Lin YP
| 1. | Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8-31, 1. |
| 2. | Visser O, Siesling S, Dijck JAAM. Incidence of Cancer in the Netherlands 1999/2000. 11th ed, Utrecht: Association of Comprehensive Cancer Centres 2003; 22-23. |
| 3. | Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000; cancer incidence, mortality and prevalence worldwide. Version 1.0. IARC CancerBase No. 5. Lyon; IARC Press. 11th ed, Utrecht: Association of Comprehensive Cancer Centres 2001; . |
| 4. | Sagar PM, Pemberton JH. Surgical management of locally recurrent rectal cancer. Br J Surg. 1996;83:293-304. |
| 5. | Frykholm GJ, Pahlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol. 1995;34:185-194. |
| 6. | van den Brink M, Stiggelbout AM, van den Hout WB, Kievit J, Klein Kranenbarg E, Marijnen CA, Nagtegaal ID, Rutten HJ, Wiggers T, van de Velde CJ. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol. 2004;22:3958-3964. |
| 7. | Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349-353. |
| 8. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. |
| 9. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. |
| 10. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. |
| 11. | Glimelius B, Gronberg H, Jarhult J, Wallgren A, Cavallin-Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol. 2003;42:476-492. |
| 12. | Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980-987. |
| 13. | Manfredi S, Benhamiche AM, Meny B, Cheynel N, Rat P, Faivre J. Population-based study of factors influencing occurrence and prognosis of local recurrence after surgery for rectal cancer. Br J Surg. 2001;88:1221-1227. |
| 14. | Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1743-1748. |
| 15. | Bakx R, van Tinteren H, van Lanschot JJ, Zoetmulder FA. Surgical treatment of locally recurrent rectal cancer. Eur J Surg Oncol. 2004;30:857-863. |
| 16. | Yamada K, Ishizawa T, Niwa K, Chuman Y, Aikou T. Pelvic exenteration and sacral resection for locally advanced primary and recurrent rectal cancer. Dis Colon Rectum. 2002;45:1078-1084. |
| 17. | Pahlman L. Population based study of factors influencing occurrence and prognosis of local recurrence after surgery for rectal cancer. Tech Coloproctol. 2003;7:120-121. |
| 18. | Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005;16:756-761. |
| 19. | Visser O, Bakx R, Zoetmulder FA, Levering CC, Meijer S, Slors JF, van Lanschot JJ. The influence of total mesorectal excision on local recurrence and survival in rectal cancer patients: a population-based study in Greater Amsterdam. J Surg Oncol. 2007;95:447-454. |
| 20. | Sobin LH, Wittekind CH. TNM Classification of Malignant Tumours. International Union Against Cancer (UICC). 6th ed. New York: John Willey & Sons 2002; . |
| 21. | Visser O, van Leeuwen FE. Stage-specific survival of epithelial cancers in North-Holland/Flevoland, The Netherlands. Eur J Cancer. 2005;41:2321-2330. |
| 22. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. |
| 23. | Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187-220. |
| 24. | Caricato M, Borzomati D, Ausania F, Valeri S, Rosignoli A, Coppola R. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126-132. |
| 25. | Temple WJ, Ketcham AS. Sacral resection for control of pelvic tumors. Am J Surg. 1992;163:370-374. |
| 26. | Bakx R, van Lanschot JJ, Zoetmulder FA. Sacral resection in cancer surgery: surgical technique and experience in 26 procedures. J Am Coll Surg. 2004;198:846-851. |
| 27. | Bakx R, van Lanschot JJ, Zoetmulder FA. Inferiorly based rectus abdominis myocutaneous flaps in surgical oncology: Indications, technique, and experience in 37 patients. J Surg Oncol. 2004;85:93-97. |
| 28. | Daland EM, Welch CE, Nathanson I. One hundred untreated cancers of the rectum. N Engl J Med. 1936;214:451-458. |