Published online Oct 21, 2008. doi: 10.3748/wjg.14.6012
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: October 21, 2008
AIM: To investigate the effects of lubiprostone and Polyethylene Glycol 3350 (PEG) on mucosal barrier repair in ischemic-injured porcine intestine.
METHODS: Ileum from 6 piglets (approximately 15 kg body weight) was subjected to ischemic conditions by occluding the local mesenteric circulation for 45 min in vivo. Ileal tissues from each pig were then harvested and mounted in Ussing chambers and bathed in oxygenated Ringer’s solution in vitro. Intestinal barrier function was assessed by measuring transepithelial electrical resistance (TER) and mucosal-to-serosal fluxes of 3H-mannitol and 14C-inulin. Statistical analyses of data collected over a 120-min time course included 2-way ANOVA for the effects of time and treatment on indices of barrier function.
RESULTS: Application of 1 μmol/L lubiprostone to the mucosal surface of ischemic-injured ileum in vitro induced significant elevations in TER compared to non-treated tissue. Lubiprostone also reduced mucosal-to-serosal fluxes of 3H-mannitol and 14C-inulin. Alternatively, application of a polyethylene laxative (PEG, 20 mmol/L) to the mucosal surface of ischemic tissues significantly increased flux of 3H-mannitol and 14C-inulin.
CONCLUSION: This experiment demonstrates that lubiprostone stimulates recovery of barrier function in ischemic intestinal tissues whereas the PEG laxative had deleterious effects on mucosal repair. These results suggest that, unlike osmotic laxatives, lubiprostone stimulates repair of the injured intestinal barrier.
- Citation: Moeser AJ, Nighot PK, Roerig B, Ueno R, Blikslager AT. Comparison of the chloride channel activator lubiprostone and the oral laxative Polyethylene Glycol 3350 on mucosal barrier repair in ischemic-injured porcine intestine. World J Gastroenterol 2008; 14(39): 6012-6017
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/6012.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6012
Ischemic intestinal disorders including intestinal volvulus, thromboembolic disease, and low flow states associated with shock, have a high mortality rate due to the rapid onset of sepsis and multiple organ failure[1-3]. Intestinal ischemic lesions are characterized by sloughing of the apical villus epithelium and rapid breakdown of mucosal barrier function[2,4,5], accompanied by increased intestinal permeability and subsequent bacterial translocation, sepsis, and multiple organ dysfunction syndrome (MODS)[6-8]. Rapid restoration of the compromised intestinal barrier is critical for patient survival. However, limited treatment options are available that target mucosal barrier repair[9].
Lubiprostone, an FDA-approved laxative (Amitiza, Sucampo Pharmaceuticals, Inc.) was previously shown to stimulate rapid repair of intestinal barrier function in ischemic-injured porcine ileum[10]. Lubiprostone activates ClC-2 Cl- channels resulting in luminal Cl- secretion and water movement responsible for its laxative properties[11-15]. ClC-2 Cl- channel activation by lubiprostone is also the predominant mechanism by which this compound stimulates repair of the tight junctions and mucosal barrier repair in ischemic tissues[10]. The mechanism for this action may relate to co-localization of ClC-2 with tight junction proteins such as occludin[16,17]. Other commercially available laxative agents such as high molecular weight Polyethylene Glycol (PEG 3350) also induce fluid movement into the lumen via different mechanisms. Polyethylene laxatives are composed of high molecular weight PEG which triggers an osmotic gradient in the lumen serving to draw ions and water from the paracellular space. In addition to its laxative effects, PEG agents have been shown to confer mucosal protective effects in various gastrointestinal injury animal models including 2, 4, 6-trinitrobenzene sulphonic acid (TNBS)-induced colitis[18] and bacterial translocation and sepsis induced by surgical stress[19].
Given the potential alternative beneficial roles of these two oral laxatives in intestinal injury and repair, the objective of this study was to compare the effects of lubiprostone and PEG 3350 on repair of mucosal barrier function in ischemic-injured intestine.
Lubiprostone was obtained from R-Tech Ueno (Sanda, Japan). PEG 3350 (Miralax, Schering-Plough Health Care Products, Inc. Kenilworth NJ) was obtained from the North Carolina State University, College of Veterinary Medicine pharmacy. 3H-mannitol and 14C-inulin were obtained from Sigma Chemical (St. Louis, MO).
All studies were approved by the North Carolina State University Institutional Animal Care and Use Committee. Six to eight-week-old Yorkshire crossbred pigs of either sex were housed individually, and maintained on a commercial pelleted feed. Pigs were fasted for 24 h prior to experimental surgery. General anesthesia was induced with xylazine (1.5 mg/kg, IM), ketamine (11 mg/kg, IM), and 5% isoflurane vaporized in 1000 mL/L O2 and was maintained with 2% isoflurane delivered via an endotracheal tube. Pigs were placed on a heating pad and ventilated with 1000 mL/L O2 using a volume-limited, time-cycled ventilator (Hallowell, Pittsfield, MA). Lactated Ringers solution was administered iv at a maintenance rate of 15 mL/kg per hour. The ileum was approached via a ventral midline incision. Ileal segments were delineated by ligating the intestine at 10-cm intervals, and subjected to ischemia by occluding the local mesenteric blood supply for 45 min.
Following the 45-min ischemic period, tissues were harvested from the pig and the mucosa was stripped from the seromuscular layer in oxygenated (950 mL/L O2/50 mL/L CO2) Ringer’s solution (mmol/L: Na+, 154; K+, 6.3; Cl-, 137; HCO3-, 24; pH 7.4) containing 5 μmol/L indomethacin to prevent endogenous prostaglandin production during the stripping procedure. Tissues were then mounted in 1.14 cm2 aperture Ussing chambers, as described in previous studies. For Ussing chamber experiments, ileal tissues from one pig were mounted on multiple Ussing chambers and subjected to different in vitro treatments. Data means are representative of 6 Ussing chamber experiments (n = 6 animals). Tissues were bathed on the serosal and mucosal sides with 10 mL Ringer’s solution. The serosal bathing solution contained 10 mmol/L glucose, and was osmotically balanced on the mucosal side with 10 mmol/L mannitol. Bathing solutions were oxygenated (950 mL/L O2/50 mL/L CO2) and circulated in water-jacketed reservoirs. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Transepithelial electrical resistance (TER) (Ω.cm2) was calculated from the spontaneous PD and short-circuit current (Isc). If the spontaneous PD was between -1.0 and 1.0 mV, tissues were current-clamped at ± 100 μA for 5 s and the PD recorded. Isc and PD were recorded at 15-min intervals over a 120-min experiment.
After tissues were mounted on Ussing chambers, tissues were allowed to acclimate for 30 min to achieve stable baseline measurements after which experimental treatments were added. Lubiprostone (1 μmol/L) or PEG 3350 (20 mmol/L) were added to the mucosal side of tissues and TER and Isc were measured at 15-min intervals over a 120-min recovery period. The PEG 3350 dose was selected as it is the recommended oral dosage for laxative properties and thus would approximate the luminal concentrations attained in vivo.
To assess mucosal permeability after experimental treatments, 0.2 μCi/mL 3H-labeled mannitol (180 kDa) and 0.2 μCi/mL, 14C-labeled inulin (5000 kDa) were added to the mucosal side of tissues mounted in Ussing chambers. After a 15-min equilibration period, standards were taken from the mucosal side of each chamber and a 60-min flux period was established by taking 0.5 mL samples from the serosal compartment. The presence of 3H and 14C was established by measuring β-emission in a liquid-scintillation counter (LKB Wallac, Model 1219 Rack Beta, Perkin Elmer Life and Analytical Sciences, Inc., Boston, MA). Unidirectional mannitol fluxes from mucosa-to-serosa were determined using standard equations.
Tissues were taken at 0 and 120 min for routine histological evaluation. Tissues were sectioned (5 μm) and stained with hematoxylin and eosin. For each tissue, 3 sections were evaluated. Four well-oriented villi and crypts were identified in each section. Villus length was obtained using a micrometer in the eye piece of a light microscope.
All data were reported as mean ± SE. TER and Isc data were analyzed by using an ANOVA for repeated measures. Radiolabeled flux data was analyzed by using a standard one-way ANOVA (Sigmastat, Jandel Scientific, San Rafael, CA). A Tukey’s test was used to determine differences between treatments following ANOVA.
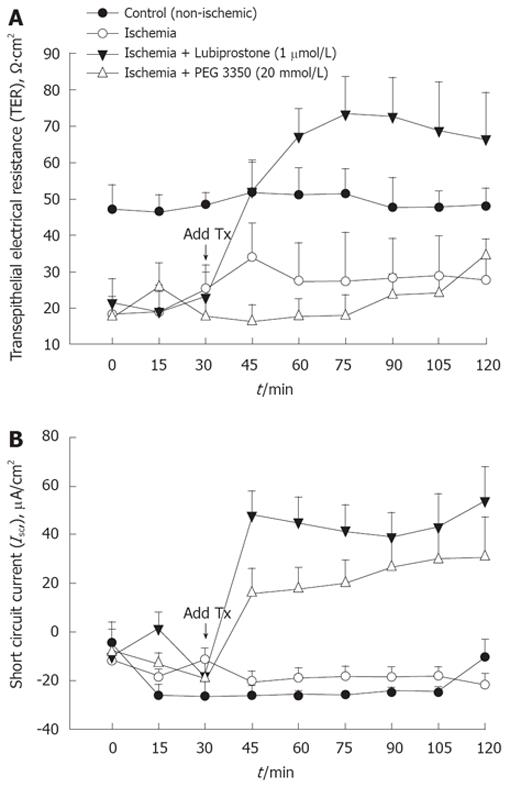
Porcine ileum was subjected to 45 min of acute mesenteric ischemia and mounted on Ussing chambers for measurement of TER and Isc over a 120-min recovery period. Ischemic-injured mucosa had significantly lower starting TER values (by approximately 40%) compared with non-ischemic control tissue (Figure 1), indicating significant impairment of intestinal barrier function induced by ischemia. Application of 1 μmol/L lubiprostone to the mucosal side of ischemic-injured mucosa induced rapid elevations in TER that attained non-ischemic control tissues within 15 min of its addition and TER continued to increase 45 min post-treatment. Lubiprostone stimulated rapid elevations in Isc (an index of electrogenic ion transport) that peaked at 15 min post-treatment (peak ΔIsc = 66 μA/cm2) and remained elevated throughout the remainder of the experiment. Mucosal addition of 20 mmol/L PEG 3350 stimulated a transient increase in TER measured 15 min after treatment; however, TER returned to ischemic control levels within 30 min post-treatment. PEG 3350 stimulated significant elevations in Isc (peak ΔIsc = 35 μA/cm2) compared with ischemic control tissues. In non-ischemic ileal tissues, lubiprostone and PEG 3350 induced similar elevations in TER (ΔTER = 30% ± 7% and 36% ± 8% in lubiprostone and PEG 3350-treated tissues) (data not shown).
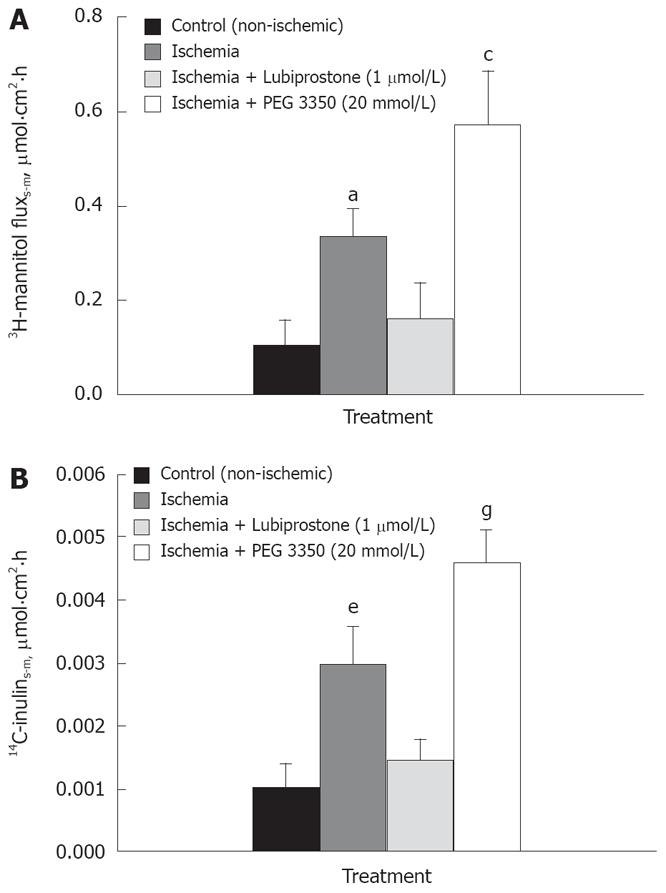
Mucosal-to-serosal flux of both medium molecular weight (3H-mannitol, 180 kDa) and large molecular weight (14C-Inulin, 5000 kDa) paracellular probes in ileal tissues mounted in Ussing chambers were conducted as an alternative measurement of mucosal permeability. In line with TER responses, ischemic tissues had greater (P < 0.01) serosal-to-mucosal fluxes of both 3H-mannitol and 14C-inulin (Figure 2). Lubiprostone treatment significantly decreased the fluxes of 3H-mannitol and 14C-inulin (P < 0.05). Alternatively, ischemic tissues treated with PEG 3350 displayed significantly increased fluxes of permeability markers compared with all other treatments (P < 0.05).
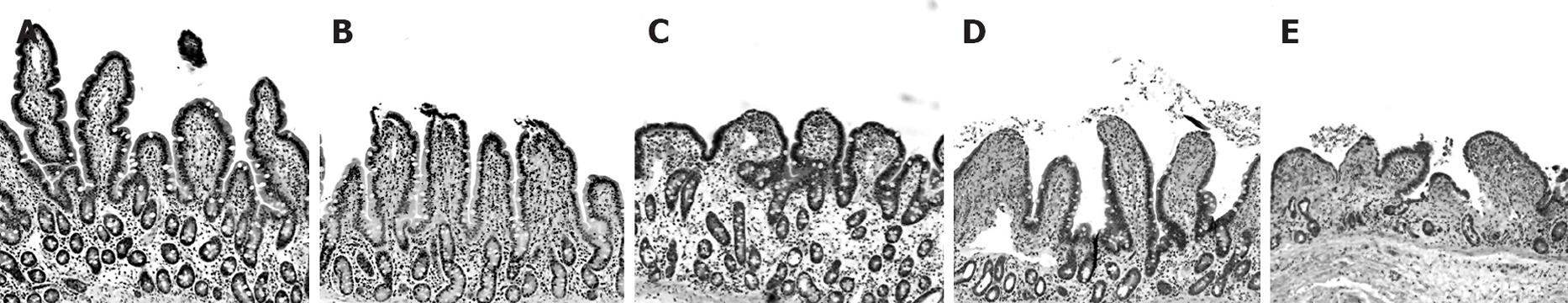
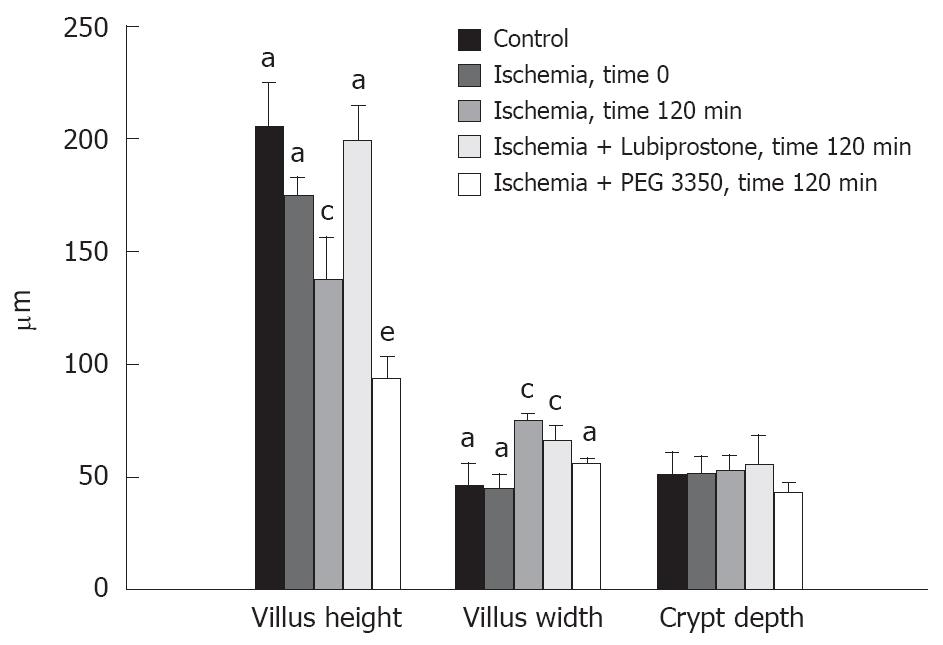
Control (non-ischemic) tissues treated with lubiprostone or PEG 3350 had no identifiable histopathological findings compared with non-treated control tissues (not shown). Ileal tissues subjected to ischemia displayed classic histological ischemic lesions characterized by sloughing of the surface epithelium of the apical villus (Figure 3). The epithelial layer was completely restituted after 60 min of mounting tissues on Ussing chambers (not shown); this effect has been demonstrated in our previous studies[4,5]. At 120 min post-ischemia on Ussing chambers, villus contraction was evident demonstrated by villi that were reduced in height and increased in width compared with non-ischemic control tissues (Figure 4). Laxative agents had a significant effect on intestinal villous length in ischemic-injured tissues in this study. Lubiprostone-treated tissues had greater villous lengths compared with ischemic-injured controls when measured at 120 min post-ischemia. In contrast, PEG 3350-treated ischemic tissues had significantly reduced villous height compared with ischemic-injured control tissues.
Results from the present study demonstrate marked differences in the ability of two oral laxatives, lubiprostone and PEG 3350, to stimulate repair of intestinal barrier function in ischemic-injured porcine ileum. Lubiprostone stimulated rapid repair of mucosal barrier function in ischemic ileal mucosa as defined by rapid elevations in TER and reductions in the mucosal-to-serosal flux of 3H-mannitol and 14C-inulin. PEG 3350 failed to induce significant changes in TER and had a detrimental influence on mucosal barrier repair evidenced by enhanced mucosal permeability to 3H-mannitol and 14C-inulin in ischemic-injured tissues treated with PEG 3350.
Oral lubiprostone and PEG 3350 both have laxative properties via different mechanisms. Lubiprostone activates ClC-2 Cl- secretion promoting Na+ and water movement into the lumen[20,21], whereas PEG 3350 induces luminal osmotic effects drawing electrolytes and water into the lumen. This is the reason that both compounds induced significant increases in short circuit current in the Ussing chambers, an indirect measure of ionic movement across the mucosa. Alternatively, there was no change in short circuit current in untreated tissues. Lubiprostone also stimulated rapid repair of mucosal barrier function in ischemic ileal tissues. Previous studies showed that lubiprostone’s influence on intestinal permeability is due to its ability to activate ClC-2 channels[10]. Although exact signaling events triggered by ClC-2 activation that lead to intestinal repair are not well understood, lubiprostone treatment was shown to trigger rapid recruitment of the tight junction protein occludin to the apical intercellular space, an event critical for the re-establishment of mucosal electrical resistance. In the present study, lubiprostone increased baseline TER in non-injured control tissues, suggesting that lubiprostone’s effect is not restricted to injured mucosa.
To our knowledge, the influence of PEG 3350 on intestinal mucosal repair has not been directly investigated. However, PEG compounds have been shown to be protective against different forms of intestinal injury. Videla et al[18] demonstrated that oral PEG 4000 was protective against TNBS-induced colitis in rats. In a study by Wu et al[19], luminal administration of a high molecular weight PEG prevented increases in intestinal permeability induced by P. aerugenosa in Caco-2 monolayers and prevented lethal sepsis in vivo induced by P. aerugenosa following surgical stress. In the latter study, PEG 4000 treatment resulted in increased mucosal hydrophobicity and reductions in baseline mucosal permeability. The reduction in permeability seen with PEG is likely attributable to its osmotic effects which may draw water from the paracellular space resulting in collapse of the tight junctions. Madara JL demonstrated that mucosal osmotic loads of 600 mosM induced rapid elevations in TER in guinea pig jejunum in Ussing chambers, an effect mediated by decreased cation selectivity of the tight junctions and alterations in the cytoskeleton[22]. In the present study, PEG 3350 induced increases in TER (by 36% ± 8%) in control (non-injured) ileal tissues. However, in the present study PEG 3350 was ineffective in improving TER in ischemic tissues and further increased mucosal permeability in ischemic-injured tissues. This suggests intact barrier function is required for PEG to stimulate increases in TER. In ischemic tissues, it is likely that PEG 3350 would freely traverse the damaged epithelium and equilibrate with the serosal compartment failing to produce an osmotic gradient in tissues.
Villous contraction in response to ischemic injury is a protective mechanism that aids in reducing the surface area of the denuded basement membrane, allowing epithelial cells adjacent to the injury to migrate and cover the epithelial defect and restore epithelial continuity[23-26]. In the present study, lubiprostone-treated ischemic ileal tissues had significantly greater villous lengths compared with other ischemic tissues. It is unclear whether this response was due to lubiprostone’s ability to inhibit villous contraction or stimulate villous lengthening during repair. Lubiprostone could have had a direct action on the principal contractile cells within the villus: myofibroblasts. These cells are arranged in linked chains of cells adjacent to the central lacteal and subjacent to the epithelial basement membrane. More studies, including a detailed time course of lubiprostone’s effects on villous architecture during recovery of ischemic injury, are required to determine this and lubiprostone’s overall relevance to intestinal barrier repair. In contrast to lubiprostone, PEG 3350-treated ileal tissues had significantly shorter villi compared with ischemic controls measured 120 min post-ischemic injury. This may represent ongoing injury in these tissues supported by increased paracellular permeability induced by PEG 3350.
Overall, this study demonstrates that lubiprostone stimulates recovery of mucosal barrier function in ischemic intestinal tissues, whereas the PEG laxative enhanced intestinal permeability. These results suggest that, unlike osmotic laxatives, lubiprostone stimulates repair of the injured intestinal barrier.
A number of important intestinal diseases, including ischemia/reperfusion injury, are characterized by damage to the epithelium lining the gut. Mechanisms are in place to rapidly repair epithelial defects, including epithelial migration (restitution). More recently, studies have shown the importance of the interepithelial tight junctions in recovery of the epithelial barrier. Studies have shown that prostaglandins and prostones increase the rate of epithelial recovery via re-assembly of tight junctions.
The prostone lubiprostone, a new medication on the market indicated for treatment of chronic constipation and irritable bowel syndrome has its effect on chloride channels (ClC-2) within tight junctions. These channels are involved in secretion of chloride in the intestine, but to a far lesser extent than the chloride channel that is genetically disrupted in patients with cystic fibrosis (CFTR). Recent studies have shown that ClC-2, when activated by prostaglandins or prostones such as lubiprostone, also play an important role in re-assembly of tight junctions, resulting in increases in the speed of epithelial repair.
Stimulation of one of the minor chloride channels, ClC-2, is an innovative way to induce low level secretion into the gut, thereby serving as a laxative. This is in contrast to traditional laxatives such as Polyethylene Glycol 3350 (PEG), which result in increased fluid in the intestinal lumen because of its osmotic properties. Other effects of ClC-2 activation by lubiprostone have recently been discovered, particularly the ability to increase mucosal repair. A comparison of lubiprostone and PEG showed that only lubiprostone facilitated the repair of the mucosa. This increased repair could not be seen at the histological level. This is consistent with prior studies showing that enhanced repair is noted at the level of tight junctions.
When treating constipation, the choice of medication includes laxatives such as PEG and the ClC-2 activator lubiprostone. The present study suggests that use of lubiprostone will also hasten the recovery of injured gut mucosa in patients with more severe intestinal disease. Further basic science research followed by clinical trials will be needed to determine the validity of these findings.
The term ClC-2 is used to describe a chloride channel in the gut epithelium that is localized to interepithelial tight junctions. The term prostone refers to a new group of compounds which are distinct from prostaglandins and specifically activate ClC-2.
The present study was performed using porcine tissues; it demonstrates that lubiprostone stimulates recovery of barrier function in ischemic intestinal tissues whereas the PEG laxative had deleterious effects on mucosal repair. This is an interesting study and well written manuscript.
Peer reviewer: Henrike Hamer, PhD, Department of Internal Medicine, Division of Gastroenterology (Box 46), Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands
S- Editor Li DL L- Editor Negro F E- Editor Ma WH
| 2. | American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000;118:951-953. |
| 3. | Boros M. Microcirculatory dysfunction during intestinal ischemia-reperfusion. Acta Physiol Hung. 2003;90:263-279. |
| 4. | Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol. 1999;276:G28-G36. |
| 5. | Blikslager AT, Roberts MC, Young KM, Rhoads JM, Argenzio RA. Genistein augments prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G207-G216. |
| 6. | Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444-451. |
| 7. | Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma. 1998;44:1031-1035; discussion 1035-1036. |
| 8. | Stechmiller JK, Treloar D, Allen N. Gut dysfunction in critically ill patients: a review of the literature. Am J Crit Care. 1997;6:204-209. |
| 9. | Blikslager AT. Treatment of gastrointestinal ischemic injury. Vet Clin North Am Equine Pract. 2003;19:715-727. |
| 10. | Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol. 2007;292:G647-G656. |
| 11. | Baker DE. Lubiprostone: a new drug for the treatment of chronic idiopathic constipation. Rev Gastroenterol Disord. 2007;7:214-222. |
| 13. | Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173-C1183. |
| 14. | Lubiprostone (Amitiza) for irritable bowel syndrome with constipation. Med Lett Drugs Ther. 2008;50:53-54. |
| 15. | Dorn SD, Ringel Y. Lubiprostone: easing the strain of constipation? Gastroenterology. 2008;134:355-357. |
| 16. | Gyomorey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol. 2000;279:C1787-C1794. |
| 17. | Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology. 2004;127:802-815. |
| 18. | Videla S, Lugea A, Vilaseca J, Guarner F, Treserra F, Salas A, Crespo E, Medina C, Malagelada JR. Polyethylene glycol enhances colonic barrier function and ameliorates experimental colitis in rats. Int J Colorectal Dis. 2007;22:571-580. |
| 19. | Wu L, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, Shapiro J, Turner JR, Wu G, Lee KY. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology. 2004;126:488-498. |
| 20. | Chang HY, Kelly EC, Lembo AJ. Current gut-directed therapies for irritable bowel syndrome. Curr Treat Options Gastroenterol. 2006;9:314-323. |
| 21. | Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345-351. |
| 22. | Madara JL. Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J Cell Biol. 1983;97:125-136. |
| 23. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. |
| 24. | Moore R, Carlson S, Madara JL. Villus contraction aids repair of intestinal epithelium after injury. Am J Physiol. 1989;257:G274-G283. |
| 25. | Erickson RA, Tarnawski A, Dines G, Stachura J. 16,16-Dimethyl prostaglandin E2 induces villus contraction in rats without affecting intestinal restitution. Gastroenterology. 1990;99:708-716. |
| 26. | Blikslager AT, Roberts MC. Mechanisms of intestinal mucosal repair. J Am Vet Med Assoc. 1997;211:1437-1441. |