Published online Oct 21, 2008. doi: 10.3748/wjg.14.5980
Revised: September 12, 2008
Accepted: September 17, 2008
Published online: October 21, 2008
AIM: To examine the role of Fibrinogen-like protein 2 (fgl2)/fibroleukin in tumor development. Fgl2 has been reported to play a vital role in the pathogenesis in MHV-3 (mouse hepatitis virus) induced fulminant and severe hepatitis, spontaneous abortion, allo- and xeno- graft rejection by mediating “immune coagulation”.
METHODS: Tumor tissues from 133 patients with six types of distinct cancers and the animal tumor tissues from human hepatocellular carcinoma (HCC) model on nude mice (established from high metastasis HCC cell line MHCC97LM6) were obtained.
RESULTS: Hfgl2 was detected in tumor tissues from 127 out of 133 patients as well as tumor tissues collected from human HCC nude mice. Hfgl2 was highly expressed both in cancer cells and interstitial inflammatory cells including macrophages, NK cells, and CD8+ T lymphocytes and vascular endothelial cells. Hfgl2 mRNA was localized in cells that expressed hfgl2 protein. Fibrin (nogen) co-localization with hfgl2 expression was determined by dual immunohistochemical staining. In vitro, IL-2 and IFN-γ increased hfgl2 mRNA by 10-100 folds and protein expression in both THP-1 and HUVEC cell lines. One-stage clotting assays demonstrated that THP-1 and HUVEC cells expressing hfgl2 had increased procoagulant activity following cytokines stimulation.
CONCLUSION: The hfg12 contributes to the hypercoagulability in cancer and may induce tumor angiogenesis and metastasis via cytokine induction.
-
Citation: Su K, Chen F, Yan WM, Zeng QL, Xu L, Xi D, Pi B, Luo XP, Ning Q. Fibrinogen-like protein 2/fibroleukin prothrombinase contributes to tumor hypercoagulability
via IL-2 and IFN-γ. World J Gastroenterol 2008; 14(39): 5980-5989 - URL: https://www.wjgnet.com/1007-9327/full/v14/i39/5980.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5980
| Diagnosis | Case | Gender | Age (yr) | Subtype | Metastasis | |
| F | M | |||||
| Colon carcinoma | 21 | 12 | 9 | 58.27 ± 10.27 | AC 21 | 17 |
| Breast cancer | 20 | 0 | 20 | 49.70 ± 11.06 | IDC 20 | 16 |
| Lung cancer | 20 | 17 | 3 | 55.17 ± 12.53 | SCC 5 | 19 |
| SqC 5 | ||||||
| AC 7 | ||||||
| AdCa 3 | ||||||
| Gastric cancer | 26 | 13 | 13 | 55.8 ± 15.88 | AC 26 | 20 |
| Esophageal carcinoma | 18 | 15 | 3 | 56.44 ± 8.63 | SqC 15 | 13 |
| AC 3 | ||||||
| Cervix cancer | 22 | 0 | 22 | 39.14 ± 6.48 | SqC 16 | 22 |
| AC 6 | ||||||
| Tumor type | Case | Hfgl2 positive | Histological localization and cases | ||||
| Tumor cell | CD57+ | CD3+, CD8+ | CD68+ | VWF | |||
| Colon carcinoma | 21 | 21 | 19 | 20 | 18 | 21 | 17 |
| Breast cancer | 20 | 20 | 18 | 20 | 20 | 20 | 18 |
| Lung cancer | 22 | 20 | 18 | 20 | 19 | 20 | 18 |
| Gastric cancer | 26 | 26 | 25 | 26 | 13 | 26 | 25 |
| Esophageal carcinoma | 19 | 18 | 18 | 16 | 15 | 18 | 14 |
| Cervix cancer | 25 | 22 | 20 | 21 | 21 | 22 | 22 |
Fibrinogen-like protein 2 (fgl2)/fibroleukin, also called fg12 prothrombinase, has recently been identified as a new member of fibrinogen-related protein superfamily, with the serine protease activity. Mouse fgl2 (mfgl2) and human fgl2 (hfgl2) are localized in chromosomes 5 and 7, respectively. The biological activity of fgl2 prothrombinase, similar to coagulating factor Xa, can directly catalyze prothrombinase into activated thrombinase, thereby, initiating a cascade coagulating reaction[1]. Several studies indicate that fgl2 is involved in MHV-3 induced fulminant hepatitis and severe or fulminant viral hepatitis in human, spontaneous abortion and xenograft rejection by mediating pathological changes such as immune coagulation, fibrin deposition, and micro-thrombus[2-5]. In addition to its primary role in homeostasis and blood coagulation, thrombin is a potent mitogen that dramatically increases the growth and metastasis potential of tumor cells. Both tissue factor (TF) and thrombin exert their influence on tumor angiogenesis and metastasis through clotting-dependent and clotting-independent pathways[6,7]. Fgl2 functions as a novel immune coagulant with the ability to generate thrombin directly. Therefore, we propose that fgl2 may contribute to tumor angiogenesis and metastasis through a clotting-dependent pathway.
In the present study, the authors investigated the expression and histological localization of hfgl2, co-localization of fgl2 with fibrin in cancer and the gene regulation of fgl2 upon cytokine induction, in the hope of providing a new point of view on the characteristic hypercoagulability of cancer and a novel anticoagulant target, the fgl2 gene.
Informed consent was obtained from all the participants, and the research protocol was reviewed and approved by the Institutional Review Board of Tongji Hospital, Wuhan, China. Patients were recruited at Tongji Hospital, and 133 tumor samples and their paired adjacent normal tissues were collected. The patients’ characteristics are shown in Table 1. The specimens for RNA extraction were frozen in liquid nitrogen until studied. Specimens for immunohistochemical and in situ hybridization were fixed in 4% paraform.
Male BALB/c-nu/nu mice (Shanghai Shilaike Animal Seed Center), 4-6 wk of age, with a body weight of 15.0-18.7 g, were kept in micro-isolated cages housed in Tongji Hospital and fed a standard lab chow diet and water ad libitum. Animals were divided into two groups: tumor-bearing mice (experimental group) and tumor-free mice (control group).
THP1 and HUVEC cell lines were purchased from Biology Treasure Center of Wuhan University. Human hepatocellular carcinoma (HCC) cell line MHCC97LM6 with high tendency of metastasis were purchased from Liver Cancer Institute, Fudan University, Shanghai. The HUVEC and MHCC97LM6 cell lines were cultured in Dulbecco modified Eagle medium (DMEM), and THP-1 cell lines were maintained in RPMI 1640 supplemented with 10% heat inactivated fetal calf serum (FCS, Gibco Life Technologies), 100 U/mL penicillin, and 100 mg/mL streptomycin and cultured at 37°C, 50 mL/L CO2, and 95% humidity.
MHCC97LM6 cell lines were cultured in vitro by sub-confluent passage in DMEM. Sub-confluent tumor cells were washed with phosphate-buffered saline (PBS), detached by a brief exposure to a 0.125% trypsin and 0.02% EDTA solution, washed in serum-containing media, and then resuspended in cold serum-free medium to get the single cell suspension. The 95% viability of the tumor cells was determined by trypan blue exclusion. The cells were kept in an ice bath until transplanted into mice. A single cell suspension of 9 × 106 cells in 100 μL serum-free media was injected subcutaneously into the dorsal scapular skin of nude mice using a 27-gauge needle. Injection with the same volume of serum-free media served as the negative control. Once a tumor was clearly visible, it was measured daily and the volume estimated by the formula V = ab2/2, where a = longest diameter, b = shortest diameter. After 36 d, the nude mice were sacrificed and the tumors and other organs including brain, heart, lung, liver, kidney, spleen, and small intestine were removed and rinsed in PBS. Aliquot of the tissue specimens were frozen in liquid nitrogen for RNA extraction. Other aliquots were fixed in 4% paraform and prepared for immunohistochemical studies. The lungs were separated into individual lobes and the number of metastatic foci was counted under a microscope with HE stain.
Immunohistochemical staining was used to assess fgl2 expression in tumor tissue and HUVEC and THP-1 cell lines. Tissues were fixed with 4% paraform, processed into paraffin, and sectioned. Then they were rehydrated with 0.1 mol/L PBS (pH 7.4) and endogenous peroxidase. Nonspecific binding was blocked by sequential incubation of the sections in 10% hydrogen peroxidase solution for 10 min followed by 10% normal goat serum in PBS at room temperature for 30 min. Thereafter tissue or cultured cell slices were incubated with a polyclonal antibody against fgl2 at a dilution of 1/300 in PBS at 4°C for 16 h. Subsequently, sections were incubated with immunoperoxidase-conjugated goat IgG fraction to rabbit IgG Fc (Zhongshan Company) at room temperature for 15 min, followed by three washes in PBS. The secondary antibody, an anti-rabbit IgG linked to peroxidase, was incubated with 3,3’-diaminobenzidine chromagen and counterstained with hematoxylin.
Fibrin was detected with the use of a rabbit-anti-fibrinogen antibody (Dako Cytomation). This reagent is known to react with fibrinogen and fibrin in mouse and human tissues. The technique used for detection of fibrin was the standard avidin-biotin complex (ABC) method. The biotinylated secondary antibody was an anti-rabbit IgG linked to peroxidase incubated with 3,3’-diaminobenzidine chromagen, followed by counterstaining with hematoxylin.
Dual staining for hfgl2 and fibrin on the same tissue was performed using a Vectastain ABC kit (Vector Laboratories), with second Abs labeled with AP or HRP, respectively.
Antibodies against CD68,CD57, CD4, CD8 and a monoclonal antibody against von Wille brand factor antigen (NeoMarkers) were individually used at a dilution of 1:50-1:100 in PBS to detect macrophages (Kupffer cells), NK cells, T lymphocytes, and vascular endothelial cells using immunoperoxidase staining via similar methodology described above.
Cells were solubilized in lysis buffer containing 10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% TritonX-100, at 4°C for 30 min. The cell lysates were subjected to centrifugation at 10 000 ×g at 4°C for 1 min. The supernatants were saved and their protein contents were measured. Thirty mg lysate protein was loaded onto 12% SDS-polyacrylamide gels. After the proteins were separated, they were transferred to a NC membrane. The membrane was blocked and probed with a polyclonal antibody against fgl2 at a dilution of 1:200 in 5% milk in TBS. After washing with TBS and 0.5% Tween-20, the blot was incubated with secondary antibodies conjugated to horseradish-peroxidase. Immunoreactive bands were detected with the enhanced chemiluminescence (ECL) reagent (Pierce).
A digoxigenin-11-UTP (Dig-UTP) (Roche)-labeled cDNA probe was cut by EcoRI following subcloning of a 169-bp fragment of mfgl2 cDNA, representing nt 756 (ACTGTGACA …) to 924 (… GAGTAAGGA), into pCR2.1 vector (Invitrogen Life Technologies). The Dig-UTP-labeled probe concentration was determined by immunoenzymatic reaction with chemiluminescent detection, and the probes were stored at -80°C. Tissue sections were deparaffinized in 100% xylene and 100% alcohol, followed by prehybridization in 50% formamide and 2 × SSC at room temperature for 1 h. The hybridization mixture consisted of 50% deionized formamide, 5% dextran sulfate, 250 μg salmon sperm DNA per milliliter, and 2 μg Dig-labeled cDNA probe per milliliter in 2 × SSC. The hybridization mixture with the probe was denatured by heating in an 85°C water bath for 5 min, chilled on ice for 1 min, and added to tissue sections for hybridization at 42°C overnight. Post-hybridization washing in a series of dilutions of SSC was followed by application of 3% blocking reagent at room temperature for 30 min. After a brief wash in Tris-HCl buffer (pH 7.5), sections were incubated with polyclonal anti-Dig Fab, conjugated to alkaline phosphatase (AP; Boehringer Manheim), and diluted 1/500 in Tris-HCl buffer. Unbound antibody was removed by two 5-min washes with Tris-HCl buffer. A purple reaction product was developed using AP substrate, 5-bromo-4-chloro-3-indolyl-phosphate, and NBT to sections at room temperature for 120 min. Sections were counterstained with methylene green and mounted with Per mount for viewing.
Total RNA was isolated from tumor specimens and cell lines using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. The concentration and purity of RNA were determined by measuring the absorbance at 260 nm and 280 nm. Subsequently, the cDNAs were synthesized. The nucleotide sequences of the primers for PCR amplification of 169 bp fragment of fgl2 were the following: sense primer, 5'-ACTGTGACATGGAGACCATG-3', and antisense primer, 5'-TCCTTACTCTTGGTCAGAAG-3'. The amplified 571 bp fragment of GAPDH cDNA was used as an internal control to ensure equal loading and first strand synthesis with forward primer, 5'-ATCACCATCTTCCAGGAG-3' and reverse primer, 5'-TGCTTCACCACCTTCTTG-3'. In the PCR reaction the DNA was amplified over 36 cycles, denatured at 94°C for 40 s, annealed at 60°C for 45 s, and extended at 72°C for 60 s. The real-time PCR reactions were performed using a SYBR green PCR kit (Biotium) in Roche Sequence Detection System. Specificity of the PCR reaction was verified by dissociation-curve analysis and agarose gel electrophoresis. Fgl2 mRNA relative quantification was assigned by reference to standard curve analysis.
THP-1 and HUVEC cell lines were maintained in medium containing 10% FBS in six well plate for 72 h until they reached sub-confluence. Then they were incubated with IL-2 (100 U/mL) or IFN-γ (200 U/mL) in medium for 4 h, 8 h, 12 h and 24 h before they were collected for immunohistochemical staining and real-time PCR studies.
Samples to be assayed for PCA were washed three times with unsupplemented RPMI 1640 and resuspended at a concentration of 106/mL. The cells were then subjected to three cycles of freeze-thawing to obtain maximal total cellular procoagulant activity. Milliunits of PCA were determined from a standard curve generated by serial log dilutions of a standard rabbit brain thromboplastin (Sigma) to determine functional shorting of the spontaneous clotting time of normal citrated human platelet-poor plasma. After addition of cellular sample, 0.1 mL of normal plasma and 0.1 mL of 25 mmol/L CaCl2 were added and clotting time was visually determined by the appearance of white precipitate after incubation at 37°C. Human plasmas deficient in specific clotting factors such as factor II or factor X (ADI/DELLWIN) were also used as substrate in the clotting assay in place of normal human plasma.
Quantitative data were expressed as mean ± SD. Statistical analysis was performed by one-way analysis of variance with P < 0.05 considered statistically significant.
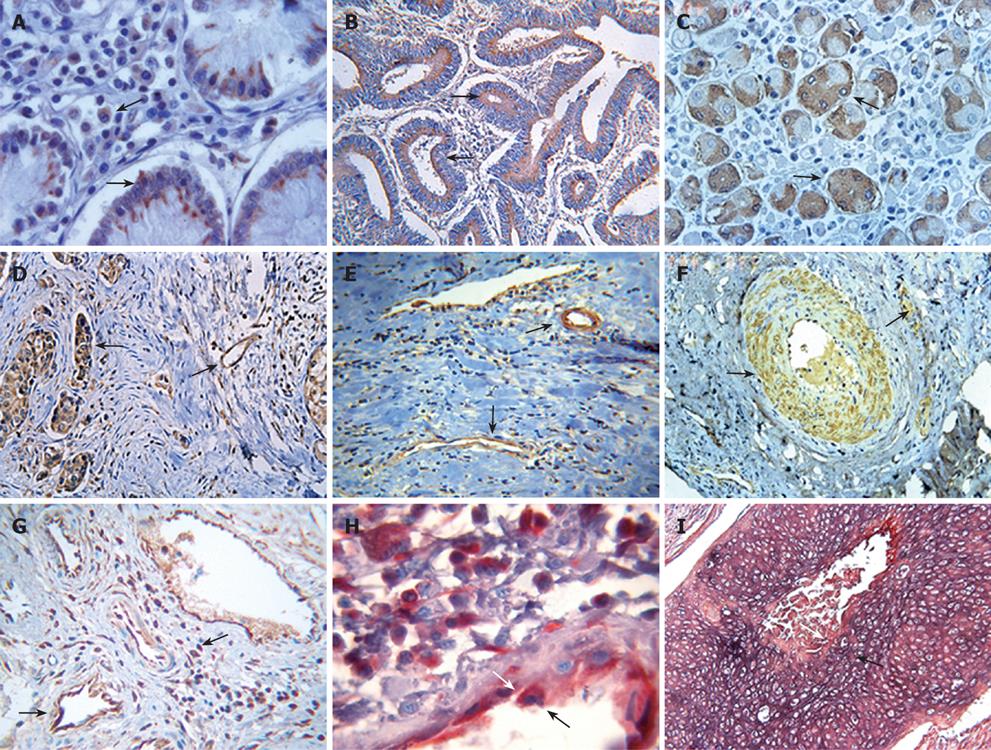
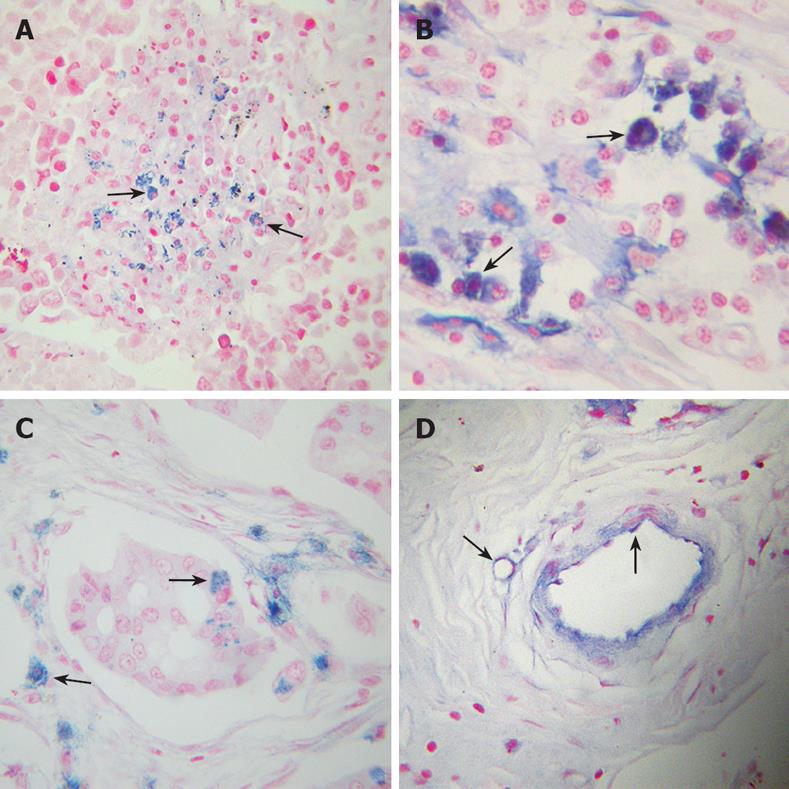
The study population was composed of 133 patients, of whom 107 patients were noted clinically to have metastasis (Table 1). Tumor tissues of the patients were examined for fgl2 expression at both the mRNA and protein levels by in situ hybridization and immunohistochemical staining respectively. The normal tissue surrounding the tumor tissue was used as control. Fgl2 was present in cancer cells as well as interstitial infiltrated and vascular endothelium cells of the microvasculature (Figure 1A-F). There was significantly upregulated hfgl2 expression with cancers when compared with those in no magnificent tumor tissues which showed little or no fgl2 expression (data not shown). Dual staining of hfgl2 and fibrin displayed the co-localization of these two molecules, indicating the contribution of highly expressed hfgl2 protein to the hypercoagulability (Figure 1G-I). In situ hybridization showed a similar pattern of hfgl2 staining in tumor tissues of the patients (Figure 2).
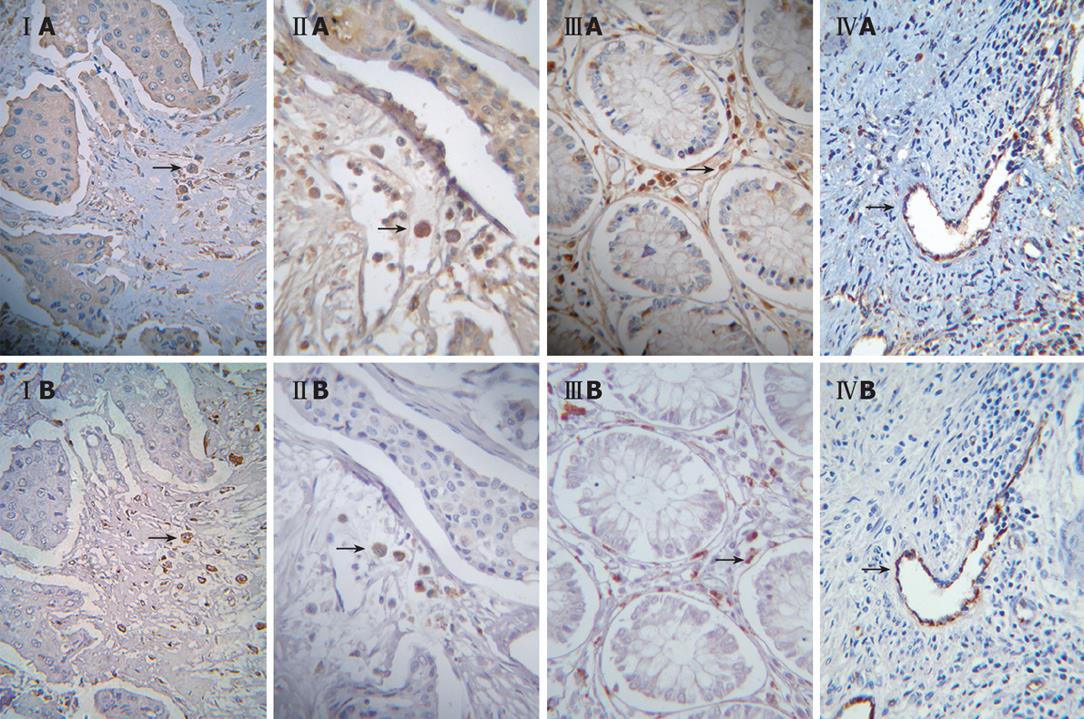
As shown with staining of serial tumor sections, the majority of CD68+, CD57+, CD8+, and vascular endothelial cells displayed increased expression of fgl2 protein in tumor tissues of the patients (Table 2 and Figure 3).
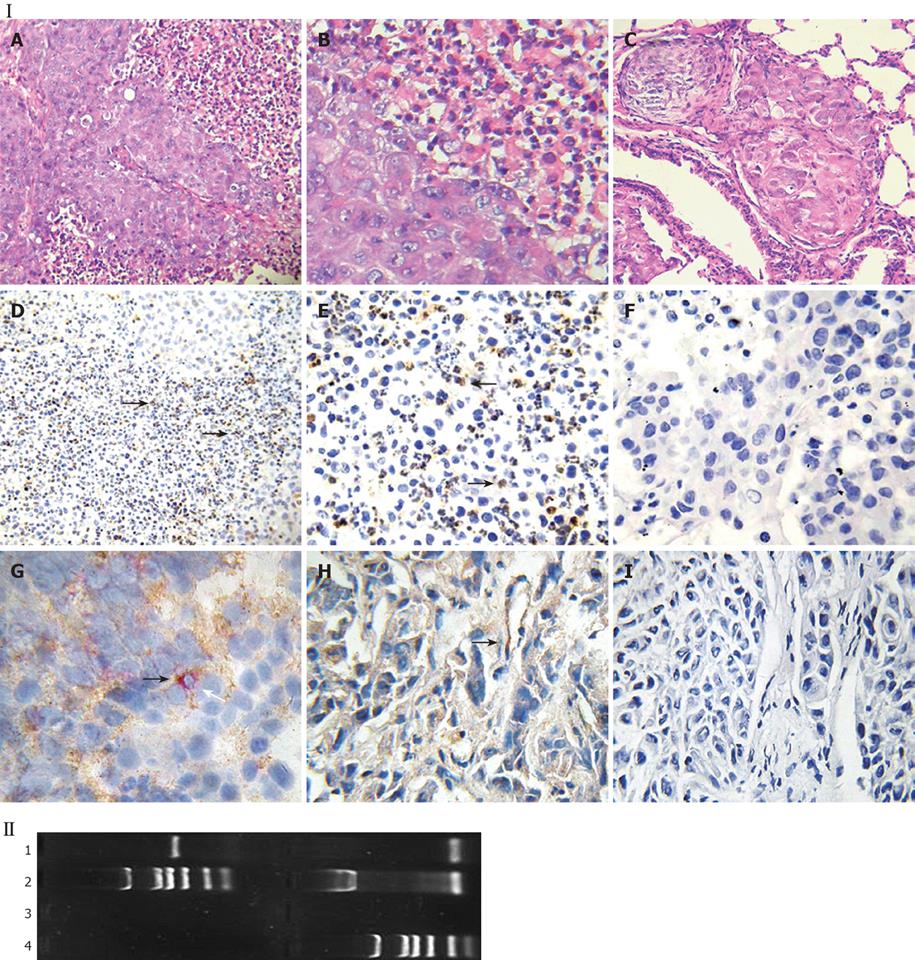
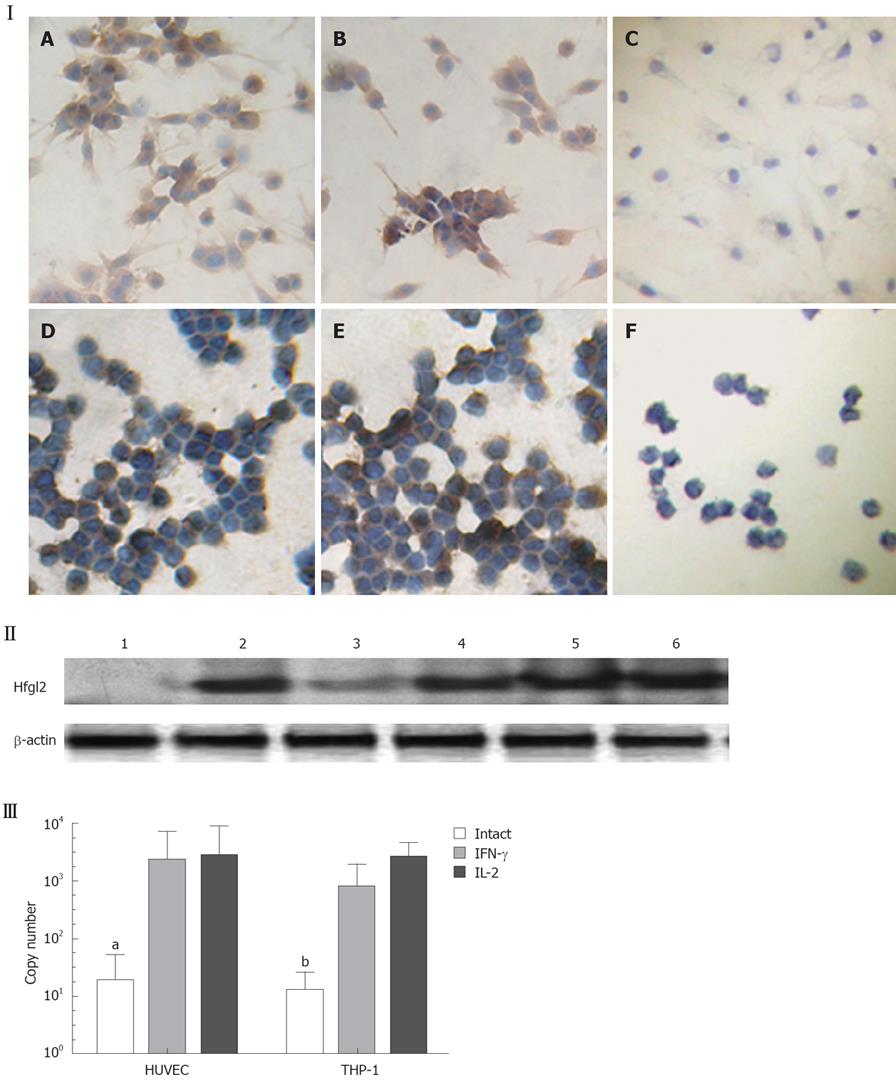
In the HCC nude mouse model, no evidence of histoincompatibility or tumor rejection was observed based on the rapid development of visible tumors after injection (100% of mice within 3 d). The steady growth of the tumors was found in MHCC97LM6 mice (Figure 4IA and B) whereas tumors were not observed in the control group. Almost all MHCC97LM6 mice developed on site palpable tumors and metastatic foci in lung tissues within 7 d of injection (Figure 4IC). Further studies showed that mfgl2 (mouse fgl2) expression in interstitial inflammatory cells and vascular endothelial cells (Figure 4ID-H). Furthermore the fgl2 was detected at mRNA level in the tumor tissue (Figure 4II).
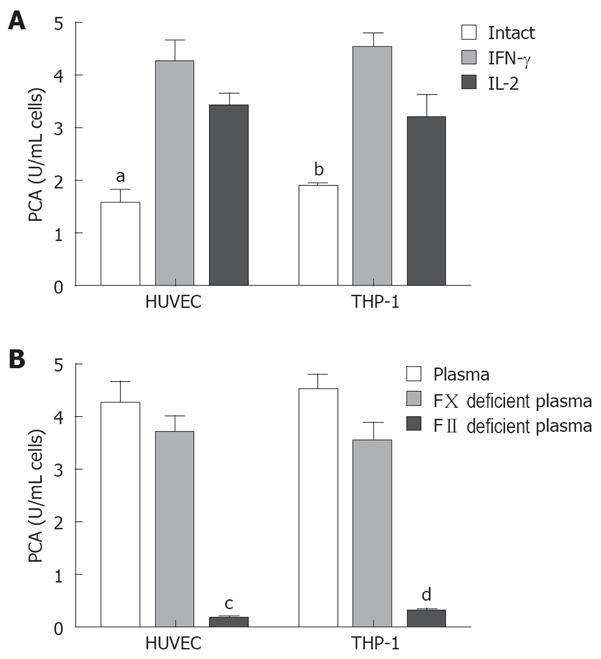
Endothelium original HUVEC and mononuclear original THP-1 cells were used to investigate the regulatory expression of fgl2 in response to various tumor cytokines involved in tumor development in vitro. RT-PCR analysis demonstrated minimal constitutive fgl2 mRNA levels in both cell lines, but increased in response to stimulation with IL-2 or IFN-γ (data not shown). This primary observation was further demonstrated by real-time PCR, which showed a 10-100 fold increase of fgl2 mRNA copies following stimulation of IFN-γ or IL-2 (Figure 5III). Immunohistochemical staining and Western-blotting also detected upregulated hfgl2 protein expression upon stimulation of cytokines (Figure 5I and II). The functional measurement of fgl2 protein was carried out by one-stage clotting assay expressed as PCA. Both HUVEC and THP-1 cells displayed basal levels of PCA with a significant increase following IL-2 or IFN-γ stimulation in parallel with fgl2 protein expression (Figure 6A). The induced PCA was independent of factor X, but closely associated with factor II, thus demonstrating the PCA was induced by increased expression of fgl2 protein (Figure 6B).
The association between thrombosis and cancer was observed by Professor Armand Trousseau in 1865, who noted that patients who present with idiopathic venous thromboembolism (VTE) frequently harbor an occult cancer. We now believe that there are two key mediators of this link: one being the thrombin[6] whose broad substrate specificity supports a variety of cellular effects relevant to tumor growth and metastasis; and the other being the tissue factor (TF)[7], the primary initiator of the coagulation cascade, whose rather ubiquitous presence as a transmembrane receptor on a variety of nucleated cells confers responsibility for the generation of cell-surface thrombin in many pathologic situations via both clotting-dependent and clotting-independent mechanisms. TF and thrombin are capable of inducing angiogenesis, the process of generating new blood vessels from preexisting vessels, which is essential for tumor growth and metastasis.
We and many others have described a new procoagulant other than tissue factor and thrombin: fgl2 prothrombinase, a member of the fibrinogen superfamily, which was primarily reported to be produced by activated macrophages, T cells, and endothelial cells. Mouse fgl2 (mfgl2) and human fgl2 (hfgl2), were localized in chromosomes 5 and 7, respectively[1,3,8]. Fgl2 is a 64-70 kDa, type 2 transmembrane protein containing a C-terminal FRED (fibrinogen related extracellular domain). The fgl2 amino acid sequence is 36% homologous to the β and γ fibrinogen chains[9]. There is 78% homology between human and mouse fgl2 with 90% homology in their C-terminal domains containing FRED[3]. Fgl2 functions as a strong prothrombinase which directly cleaves prothrombin to thrombin leading to fibrin deposition in the absence of factor VII or factor X[10]. The direct prothrombinase activity of fgl2 is implicated in the pathogenesis of several inflammatory disorders including fulminant hepatitis and severe hepatitis, allo- and xeno-graft rejection[4,11,12]. Furthermore, its role is also evidenced in murine and human cytokine induced fetal loss[5,13-15] and neonatal death from contractile dysfunction and rhythm abnormalities during embryonic and postnatal development[16]. The observations that neutralizing Abs to mfgl2 prevent both fibrin deposition and death from MHV-3 infection support its role as a coagulant[17]. Recent studies have shown that inhibition of reticuloendothelial cell mfgl2 expression through the use of gene-targeted fgl2-deficient (fgl2-/-) mice or targeted fgl2 gene with antisense mfgl2 results in the prevention of MHV-3-induced fibrin deposition, liver injury, and death[2,18].
Our study shows that fgl2 prothrombinase was expressed in malignant tumor tissues including colon, breast, lung, gastric, esophageal, and cervical tissues from patients and in HCC nude mouse models. Up-regulation of fgl2 gene expression is evident not only in cancer cells, but also in interstitial infiltrated cells including macrophages, NK cells, CD8+ T lymphocytes, and vascular endothelial cells. Dual staining shows that fibrin (nogen) uniformly co-localized with fgl2 protein. In breast cancer, fgl2 is present predominantly in the same cellular types in which TF was expressed[19]. Other studies have further shown fibrin (nogen) co-localization with TF expression. Cross-linked fibrin (XLF) was found within the endothelium of angiogenic vessels of invasive breast cancer specimens, but not within the vessels of benign breast tumors in histological specimens from the patients[20]. The similar expression patterns of TF and fgl2 have led us to hypothesize that both fgl2 and TF may be responsible for the coagulation cascade in cancer. Fgl2 and TF cleavage of prothrombin to thrombin results in fibrin deposition in the tumor microenvironment (TME). Thrombin-catalyzed, XLF formation is a characteristic histopathological finding in many human and experimental tumors[21].
Fgl2 induces angiogenesis by generating thrombin. Thrombin dramatically increases the growth and metastatic potential of tumor cells via clotting dependent and independent mechanisms. The fibrin matrix that develops around tumors provides a provisional proangiogenic scaffold that supports vessel formation and stimulates endothelial cell proliferation and migration through clotting dependent mechanisms. Clotting independent mechanisms are thought to be mediated via proteolytic cleavage of the PARs and subsequent activation of G-protein-coupled signal transduction cascades, leading to the upregulation of many angiogenesis-related genes, including VEGF, VEGF receptors, TF, bFGF, and MMP-2[22-24]. These genes can create a number of pleiotropic responses, such as change in endothelial cell shape, increased vascular permeability, increased endothelial cell proliferation, and increased proteolysis, all of which contribute to increased tumor angiogenesis.
The pathogenic role of fgl2 is not entirely understood as only one pathway of fgl2 activation has been studied so far. In murine hepatitis viral infection, nucleocapsid protein induces transcription of fgl2 through the transcription factor hepatic nuclear factor 4α and its cognate receptor[25,26]. HBV X and core protein was shown to induce hfgl2 expression through a host factor c-Ets-2 and MAPK signal pathway[27]. In transplantation, fgl2 transcription appears to be regulated by cytokines. Macrophage induction of fgl2 is induced by IFN-γ, whereas preliminary data suggest that fgl2 transcription in endothelial cells occurs in response to TNF-α but not IFN-γ[28].
Our study has also shown that cultured HUVEC and THP-1 cells activated by IFN-γ or IL-2 demonstrated induction of hgl2 expression and enhanced activation of human prothrombin. The induced PCA activity was independent of factor X, but closely associated with factor II. These results suggest that macrophages are attracted to invading tumors and subsequently release cytokines that later induce fgl2 expression in cancer. Increased fgl2 expression may activate thrombin, to exert its effect on tumor angiogenesis and metastasis through clotting-dependent and independent mechanisms. Additional studies in molecular pathways for induction of fgl2 in cancer are presently underway in our laboratory.
The fgl2 protein described here is a membrane bound prothrombinase. The recent discovery of a secreted form of fgl2 (sfgl2) produced by T regulatory cells has potent immune modulatory effects on the adaptive immune system. Sfgl2 was reported to prevent maturation of dendritic cells (DC) by inhibiting NF-κB nuclear translocation, expression of CD80 and MHCII, by inhibiting T cell proliferation in response to CD3/CD28, Concanavalin A, and allo-antigens. These observations have provided a potential explanation for many of the biological functions influenced by fgl2 protein[29-31] in our laboratory. fgl2 was also found in the extracellular matrix in malignant tumor tissue samples. This suggests the involvement of sfgl2 protein. Further studies are necessary to solve this conundrum.
In this study, we first reported the highly expressed fgl2 prothrombinase in a variety of tumor tissues both from patients and an animal model. Tumor related cytokines IFN-γ and IL-2 lead to the induction of hfgl2 expression and enhanced activation of human prothrombin. These observations suggest that fgl2 prothrombinase, in conjunction with thrombin and tissue factor, may contribute to tumor hypercoagulability and possibly to angiogenesis and metastasis. In turn, fgl2 may serve as a novel target for intervention of tumor development.
Fibrinogen-like protein 2 (fgl2)/fibroleukin, also called fg12 prothrombinase, has been found recently and belongs to fibrinogen-related protein superfamily. Fgl2 prothrombinase has serine protease activity. Human fgl2 gene is mapped at chromosome 7q11,23. Biological activity of the product of fgl2 prothrombinase, similar to coagulating factor Xa, can directly catalyze prothrombinase into activated thrombinase, initiating cascade coagulating reaction. Several studies abroad indicate that mouse fgl2 has been involved in MHV-3 induced fulminant hepatitis, spontaneous abortion and xenograft rejection by mediating “immune coagulation”, fibrin deposition and microthrombus leading to the pathological changes.
In addition to its primary role in hemostasis and blood coagulation, thrombin is a potent mitogen capable of inducing cellular functions. Thrombin can dramatically increase the growth and metastatic potential of tumor cells, thus it should be of great importance in the behavior of cancer. Both tissue factor (TF) and thrombin exert their influence on tumor angiogenesis and metastasis through clotting-dependent and clotting-independent mechanisms. Fgl2 functions as a novel immune coagulant with the ability to generate thrombin directly.
Fgl2 highly expressed in tumor cells and activated interstitial infiltrated cells, which may contribute to the characteristics of hypercoagulability and in turn induces tumor angiogenesis and metastasis.
In present study, the authors investigated hfgl2 expression and its histological localization in cancer, which will provide a new point of view on the characteristic hypercoagulability of cancer and efficacious anticoagulant therapy in cancer treatment.
It has been proved that fgl2 functions as an immune coagulant with the ability to cleave prothrombin to thrombin directly and there are relationships between thrombosis and cancer. The aim of this study was to investigate the role of fgl2 in tumor development. They found that Hfgl2 was detected in tumor tissues from 127 out of 133 patients as well as tumor tissues collected from human HCC nude mice and IL-2 and IFN-γ could increase hfgl2 mRNA in vitro. It is an interesting subject and results were clearly described.
Peer reviewer: Xin-Xin Zhang, Professor, Department of Infectious Diseases, Rui Jin Hospital, 197, Rui Jin Er Road, Shanghai 200025, China
S- Editor Zhong XY L- Editor Ma JY E- Editor Lin YP
| 1. | Levy GA, Liu M, Ding J, Yuwaraj S, Leibowitz J, Marsden PA, Ning Q, Kovalinka A, Phillips MJ. Molecular and functional analysis of the human prothrombinase gene (HFGL2) and its role in viral hepatitis. Am J Pathol. 2000;156:1217-1225. |
| 2. | Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, Ghanekar A, Scott JA, Miller T, Chan CW. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58-66. |
| 3. | Ding JW, Ning Q, Liu MF, Lai A, Peltekian K, Fung L, Holloway C, Yeger H, Phillips MJ, Levy GA. Expression of the fgl2 and its protein product (prothrombinase) in tissues during murine hepatitis virus strain-3 (MHV-3) infection. Adv Exp Med Biol. 1998;440:609-618. |
| 4. | Zhu CL, Yan WM, Zhu F, Zhu YF, Xi D, Tian DY, Levy G, Luo XP, Ning Q. Fibrinogen-like protein 2 fibroleukin expression and its correlation with disease progression in murine hepatitis virus type 3-induced fulminant hepatitis and in patients with severe viral hepatitis B. World J Gastroenterol. 2005;11:6936-6940. |
| 5. | Clark DA, Arck PC, Chaouat G. Why did your mother reject you? Immunogenetic determinants of the response to environmental selective pressure expressed at the uterine level. Am J Reprod Immunol. 1999;41:5-22. |
| 6. | Maragoudakis ME, Tsopanoglou NE, Andriopoulou P. Mechanism of thrombin-induced angiogenesis. Biochem Soc Trans. 2002;30:173-177. |
| 7. | Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73:145-150. |
| 8. | Yuwaraj S, Ding J, Liu M, Marsden PA, Levy GA. Genomic characterization, localization, and functional expression of FGL2, the human gene encoding fibroleukin: a novel human procoagulant. Genomics. 2001;71:330-338. |
| 9. | Doolittle RF. The structure and evolution of vertebrate fibrinogen: a comparison of the lamprey and mammalian proteins. Adv Exp Med Biol. 1990;281:25-37. |
| 10. | Schwartz BS, Levy GA, Fair DS, Edgington TS. Murine lymphoid procoagulant activity induced by bacterial lipopolysaccharide and immune complexes is a monocyte prothrombinase. J Exp Med. 1982;155:1464-1479. |
| 11. | Ghanekar A, Mendicino M, Liu H, He W, Liu M, Zhong R, Phillips MJ, Levy GA, Grant DR. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004;172:5693-5701. |
| 12. | Mendicino M, Liu M, Ghanekar A, He W, Koscik C, Shalev I, Javadi M, Turnbull J, Chen W, Fung L. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112:248-256. |
| 13. | Clark DA, Foerster K, Fung L, He W, Lee L, Mendicino M, Markert UR, Gorczynski RM, Marsden PA, Levy GA. The fgl2 prothrombinase/fibroleukin gene is required for lipopolysaccharide-triggered abortions and for normal mouse reproduction. Mol Hum Reprod. 2004;10:99-108. |
| 14. | Knackstedt M, Ding JW, Arck PC, Hertwig K, Coulam CB, August C, Lea R, Dudenhausen JW, Gorczynski RM, Levy GA. Activation of the novel prothrombinase, fg12, as a basis for the pregnancy complications spontaneous abortion and pre-eclampsia. Am J Reprod Immunol. 2001;46:196-210. |
| 15. | Knackstedt MK, Zenclussen AC, Hertwig K, Hagen E, Dudenhausen JW, Clark DA, Arck PC. Th1 cytokines and the prothrombinase fgl2 in stress-triggered and inflammatory abortion. Am J Reprod Immunol. 2003;49:210-220. |
| 16. | Mu J, Qu D, Bartczak A, Phillips MJ, Manuel J, He W, Koscik C, Mendicino M, Zhang L, Clark DA. Fgl2 deficiency causes neonatal death and cardiac dysfunction during embryonic and postnatal development in mice. Physiol Genomics. 2007;31:53-62. |
| 17. | Li C, Fung LS, Chung S, Crow A, Myers-Mason N, Phillips MJ, Leibowitz JL, Cole E, Ottaway CA, Levy G. Monoclonal antiprothrombinase (3D4.3) prevents mortality from murine hepatitis virus (MHV-3) infection. J Exp Med. 1992;176:689-697. |
| 18. | Zhu C, Sun Y, Luo X, Yan W, Xi D, Ning Q. Novel mfgl2 antisense plasmid inhibits murine fgl2 expression and ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther. 2006;17:589-600. |
| 19. | Shoji M, Hancock WW, Abe K, Micko C, Casper KA, Baine RM, Wilcox JN, Danave I, Dillehay DL, Matthews E. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399-411. |
| 20. | Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209-215. |
| 21. | van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426-437. |
| 22. | Richard DE, Vouret-Craviari V, Pouyssegur J. Angiogenesis and G-protein-coupled receptors: signals that bridge the gap. Oncogene. 2001;20:1556-1562. |
| 25. | Ning Q, Liu M, Kongkham P, Lai MM, Marsden PA, Tseng J, Pereira B, Belyavskyi M, Leibowitz J, Phillips MJ. The nucleocapsid protein of murine hepatitis virus type 3 induces transcription of the novel fgl2 prothrombinase gene. J Biol Chem. 1999;274:9930-9936. |
| 26. | Ning Q, Lakatoo S, Liu M, Yang W, Wang Z, Phillips MJ, Levy GA. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4 alpha. J Biol Chem. 2003;278:15541-15549. |
| 27. | Han MF, Zhou YY, Xi D, Yan WM, Luo XP, Ning Q. Hepatitis B virus proteins induce activation of hfgl2 transcription through cEts2 transcription factor and MAPK signal pathway. Hepatology. 2007;46 Suppl:641A. |
| 28. | Hancock WW, Szaba FM, Berggren KN, Parent MA, Mullarky IK, Pearl J, Cooper AM, Ely KH, Woodland DL, Kim IJ. Intact type 1 immunity and immune-associated coagulative responses in mice lacking IFN gamma-inducible fibrinogen-like protein 2. Proc Natl Acad Sci USA. 2004;101:3005-3010. |
| 29. | Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. J Immunol. 2003;170:4036-4044. |