INTRODUCTION
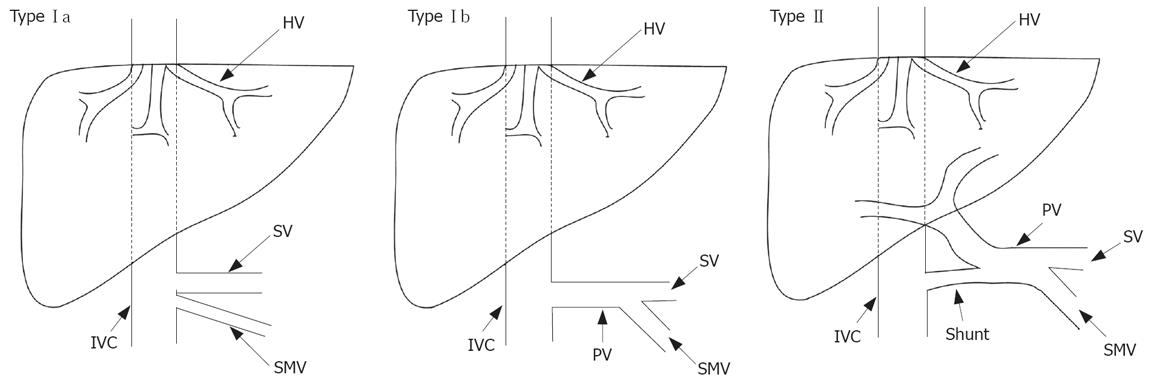
Figure 1 Schematic demonstration of different types of Abernethy abnormality[2].
SMV: Superior mesenteric vein; HV: Hepatic vein; SV: Splenic vein; PV: Portal vein; IVC: Inferior vena cava.
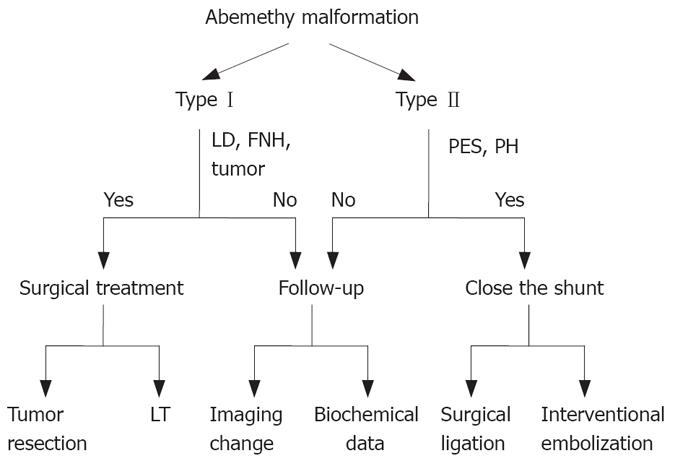
Figure 2 Schematic demonstration of decision-making advices for treatment of Abernethy malformation.
LD: Liver dysfunction; FNH: Focal nodular hyperplasia; PSE: Porto-systemic encephalopathy; PH: Portal hypertension; LT: Liver transplantation.
The first account of congenital absence of the portal vein (CAPV) was given by John Abernethy in 1793[1], based on a postmortem examination of a 10-mo-old female, which revealed the termination of portal vein in the inferior vena cava at the insertion level of the renal veins and multiple congenital abnormalities other than CAPV. It was reported that complete portosystemic shunts not perfusing the liver via portal vein are defined as type I, whereas partial shunts with a remaining degree of portal perfusion to the liver are defined as type II[2]. Furthermore, type I is sub-classified into types Ia and Ib depending on the anatomy of portal vein. In type Ia, the splenic vein (SV) and superior mesenteric vein (SMV) drain separately in type Ia, while both drain together in type Ib after uniting to form a common trunk[3]. Howard and Davenport[4] suggested that the congenital diversion of portal blood away from the liver, by either an end-to-side or a side-to-side shunt, is known as the Abernethy malformation. Thus, CAPV associated with extra hepatic portocaval shunts can be referred to as an Abernethy type I malformation (Figure 1).
ANATOMY AND EMBRYOLOGY
The portal vein returns blood from the intraperitoneal section of the gastrointestinal tract and the spleen, pancreas, and biliary apparatus, while SV and SMV return blood form the portal vein. At the porta hepatis, the portal vein is subdivided into right and left branches (besides providing the quadrate lobe with an additional branch). These branches ramify to form small vessels that drain into the sinusoids[5]. CAPV with an extrahepatic porto-caval shunt means that the mesenteric vasculature where splanchnic blood bypasses the liver through a congenital shunt vessel, completely drains into the systemic circulations, such as inferior vena cava, left renal vein, right atrium, iliac vein, left hepatic vein (HV), and azygos vein[6-8]. Strictly speaking, CAPV is also characterized by complete absence of venules within the portal areas, which has been confirmed by liver biopsy[9,10]. The case seems to be a complete portal vein agenesis[10]. Similarly, complete absence of the portal vein with intrahepatic portal venules can be named portal vein atresia.
The portal vein develops embryologically between the 4th and 10th weeks[10,11]. In the 4th week embryo, three paired venous systems are present: umbilical veins of chorionic origin, vitelline veins from the yolk sac and cardinal veins from the body of the embryo. Towards the end of the 4th week, three cross links are formed between the right and left vitelline veins[12]. The intrahepatic portal veins develop from the superior link, while the extrahepatic portal vein forms during the selective involution of the caudal part of the right and left vitelline veins. Selective involution of these communications generates the fully developed portal vein[11]. Primary failure to form this critical anastomosis will lead to complete or partial absence of the portal system. As a result, mesenteric and splenic venous flow cannot but drain into renal veins, HVs, or directly into the inferior vena cava (IVC)[6-8,13], with relatively poor perfusion of the liver[14].
CLINICAL MANIFESTATIONS
CAPV can cause a broad spectrum of clinical manifestations, which can be divided into two groups: concomitant congenital abnormalities and sequent syndromes. The former can be referred to as a congenital hepatopathy and a congenital cardiopathy, which can be explained by its close relation to the development of cardiovascular system.
Systemic shunting of the visceral venous return can lead to abnormal hepatic development, function and regeneration secondary to the absence of portal hepatotrophic factor, resulting in the development of focal nodular hyperplasia (FNH) and hepatic tumors[15-17]. Other than CAPV, many cases are presented with liver abnormalities, including FNH[7,15,18-30] or nodular regenerative hyperplasia (NRH)[8,31-35]. Several patients have been found to have combined hepatocellular adenoma[31,36,37]. These are generally considered benign parenchymatous lesions, and also have the potential to deteriorate into hepatoblastoma[26,28] and hepatocellular carcinoma (HCC)[12,20,38,39]. CAPV, though rare, could be found along with chronic hepatitis[20] and liver cirrhosis[17]. Gocmen et al[40] reported a 7-year-old boy with congenital hepatic fibrosis (CHF). Intrahepatic calcification was detected during prenatal diagnosis in a fetus. The liver volume is often larger than normal because of the regenerative or hyperplasia nodular change and sometime hepatomegaly might be observed[15]. Moreover, the mass can cause a series of mechanical pressure symptomes, such as mild intermittent jaundice and pruritus[35]. Nevertheless, the liver size may be small due to insufficient portal vein supply or lobular hypoplasia[41,42]. Abnormal hepatic development, with aberrant lobation, absence of ligamentum teres and falciform ligament, has been noted in Hellwegg’s patients[43]. Liver dysfunction (LD), frequently found in liver tumors, also occurs in patients without liver mass.
CAPV may result from a embryologic insult which causes defect of the cardiovascular system and complicated cardiogenesis could be affected by the insult or the systemic diversion of portal venous flow[11]. Congenital cardiac diseases including atrial septal defect (ASD)[2,21,26,28,37,44,45] or patent foramen ovale[43], ventricular septal defect (VSD)[16,17,21,26,31,40], and patent ductus arterious (PDA)[12,23,43,44] are frequently observed along with CAPV. Bellah et al[45] have also detected a fatal congenital hypertrophic cardiomyopathy with ultrasound in an infant. Dextrocardia[1] and Mesocardia[44] that reported congenital cardiac abnormalities are rarely found in CAPV cases. Congenital stenosis of aortic valve[11] and pulmonary artery valve[45], found in a few CAPV cases, can cause tricuspid regurgitation[44]. Even stenosis[11] or coarctation[12] has been observed in aorta. Most frequently encountered Cardiovascular lesions, most frequently encountered in CAPV patients with Goldenhar syndrome, are tetralogy of Fallot and VSD[46]. Cardiomegaly has been noted in CAPV patients[41,47], which may result from congenital insult or increased blood flow into the heart with a certain degree of congestive heart failure. A large number of non-heart abnormalities are found in all the cases reviewed[3,9,14,27,34-36,39,48,49].
Besides CAPV, visceral or cutaneous vascular malformations have been reported, such as double SV[1], double inferior vena cava[40], left sided IVC[2,17,26], hepatic artery originating from superior mesenteric artery[8,50], intrapulmonary shunting[7], azygos and hemiazygos continuation[1,17,43,48], and skin hemangioma[14,43]. Obvious compensatory changes may be found in blood vessels, especially in veins returning visceral blood or ramus anastomoticus, such as the SV[51], inferior mesenteric vein[33,52], left renal vein[44,53], azygos system[30,51], IVC[14,44,53], and right atrium[14]. Hepatic artery is enlarged and hypertrophied[1,19] due to the same reason.
Polysplenia[1,2,4,17,26,54], megalosplenia[14,24,51] and hypersplenism[51] are the frequently encountered splanchnic abnormalities other than hepatic and cardiac abnormalities, which may be due to the embryonic impairment or portal hypertension (PH). The latter is a fault because CAPV accompanying PH has merely been observed in several cases[9,16,55], which is inconsistent with our general deduction. One reasonable explanation is out of the congenital adaptation.
It was reported that CAPV patients could also have congenital biliary atresia (CBA)[2,4,17,21], congenital choledochal cyst[29], and intrahepatic gallbladder[43]. The urinogenital system including cystic dysplasia of the kidneys[22,24], bilateral ureteropelvic obstruction of the kidneys[45], vesicoureteral reflux[33], crossed fuses renal ectopia[33,40], and hypospadias[56], is also involved. Nonfunctioning pancreatic tumor[25], ulcerative colitis(UC)[33], juvenile polyposis[21], inguinal hernia[23,37], and even situs inversus viscerum[2,4], are sporadically observed in CAPV patients.
The skeletal systematic abnormalities, such as radial hypoplasia[33] and congenital absence of the first metacarpophalangeal complex of the right hand[33], are noted in Grazioli’s patients. Vertebral abnormalities or hemivertebra scoliolosis[15,40,57], and oculoauriculovertebral dysplasia or Goldenhar’s syndrome[16,26,28,58] exhibit thoracic hemivertebrae, right maxillary hypoplasia, mild micrognathia, and short fifth fingers, are not uncommon in CAPV patients.
Sequent syndromes, which can result in poor prognosis[27], are mainly related to LD or liver abnormalities besides CAPV malformation. Although most cases do not possess any liver abnormalities[1,3,9,27,43-45,47-49,54,57], CAPV patients suffer from different levels of LD[2-4,9,15,16,20,23,24,26-29,34,37,38,44,47,55,56] possibly due to the lack of portal flow. Hepatic encephalopathy, hepatopulmonary syndrome (HPS), and hepatorenal syndrome are closely related to metabolic disorder because of liver lesions, including hyperammonemia[3,27,41,59] and galactosemia[20,27]. Toxic compounds produced in the digestion process only bypass the liver into the systemic circulation in CAPV patients, and are prone to cause hepatic encephalopathy[23,26,27,34,40,49,56]. Mild CAPV patients present with cognitive retardation[33] or mental retardation[27], and their symptoms are merely drowsiness or delirium[56]. Wakamoto et al[23] reported the first case of sub-clinical porto-systemic encephalopathy (PSE) with CAPV. When it becomes worse, tremor or orthostatic disturbance[56], abscess in the brain[60], even epilepsy[38] and cerebellar meningioma[55] may occur. If the porto-systematic shunt ratio is high enough, HPS[27,30,60-63] may occur, including cyanosis[30] of the hands, feet, and lips, digital clubbing and pectus excavatum[60], bronchial asthma[8,23] or hypoxemia-induced bronchial stenosis[56], pulmonary hypertension[56,64]. When the shunt ratio is over 90%, CAPV patients would have hematuria and proteinuria, namely hydropigenous nephritis[65]. CAPV patients also could have chronic renal failure[33,34]. Gonadal hormonal disorder[33,47,66] can result in hypergalactocemia[66-68], primary amenorrhoea and signs of virilization[33]. Satoh’s patients present with hyperandrogenism, insulin resistant hyperinsulinaemia, and hyperglycaemia[47]. A few cases of PH[9,16,55], rectal bleeding[9,21,33,42], anaemia[31,33] and peripheral edema[21] have also been reported. All the metabolic disorders described above can lead to growth retardation[21,40,56], small head or microcephaly[14,38].
Routine clinical examination can find the above or other diseases in CAPV patients, suggesting that there are a large number of asymptomatic CAPV patients.
ETIOLOGY
Cardiovascular system
Embryologically, paired vitelline veins enter the embryo with yolk stalk, anastomose with each other around the developing duodenum forming a loop, and pass through the septum transversum to the sinus venosus. Portal venous system development occurs depending on selective apoptosis of the bilaterial vitelline veins and their median links before entering the septum transversum[33]. The whole process is complex and coinciding, any insult may affect the development resulting in a preduodenal portal vein, CAPV and duplications, as well as communications between the portal and pulmonary veins[10,12,16,28]. The pathogenesis of CAPV may be attributed to excessive involution of the peri-intestinal vitelline venous loop[10,13,22,69], or to total failure of the vitelline veins to establish the critical anastomosis with hepatic sinusoids[4]. Behind the abnormalities, the initiative event may be referred to genetic mutation or chromatosome variation as CAPV has been sometimes reported in conjunction with chromosomal disorders[70], such as translocation (2,10)[21] and turner syndrome (45, XO)[6,71]. The associated extrahepatic portosystemic shunts may occur due to the persistent subcardinohepatic anastomosis with the vitelline veins. The subcardinohepatic anastomosis connects the vitelline vein that develops into the portal system and the right subcardinal vein that develops into the renal segment of the IVC, as well as forms the hepatic segment of the IVC, thus accounting for the high incidence of draining points at the suprarenal IVC[3].
Cardiac malformations are frequently observed in patients with CAPV[11,72], the close relationship between the development of vitelline veins and the heart in embryonic life may be responsible for the association between cardiovascular malformations and CAPV[12,40,45]. The cardiac abnormalities may result from a some embryogenic insults and compensate for the congestive effect of portal vein absence and shunting[37], indicating that prenatal insult occurs during the concurrent development of the heart and gastrointestinal tract. However, it was also supposed that systemic shunt of the portal venous flow could adversely affect the hepatic and cardiac development and function[11]. It was reported that concomitant atrial and ventricular septal defects related to CAPV may be attributed to a congenital adaptive change occurring during the development from the embryonic stage, which tends to compensate for the congestive effects of portal venous aplasia[10]. CAPV can result in cardiomegaly or even congestive heart failure due to the shunts of blood flow[10].
Liver
The importance of intact portal vein flow following liver resection or transplantation has been recognized both in experiments and in clinical practice[46,73]. The lack of portal flow can affect the development, function and regenerative pability of the liver. The importance of certain substances, such as insulin and glucagon, is underscored because of CAPV[73], and these substances are no longer supplied to the liver through the mesenteric blood flow and lead to hepatic hypoplasia because they help maintain the hepatic structure and function[74-77]. The hepatic volume is extremely small compared with the standard one as detected by computed tomography (CT) volumetry[56]. However, as far as the regenerative ability of the liver is concerned, such an assumption is questionable. In fact, in cases of CAPV, the regenerative ability appears to be normal after liver lobectomy and trisegmentectomy[16,37].
CAPV is frequently observed with hepatic tumors and tumor-like conditions, such as FNH and NRH[40], suggesting that intrahepatic changes due to hemodynamic imbalance participate in the development of liver tumors[8]. In CAPV patients, liver is supplied only by the hepatic artery in the absence of the portal vein[18]. Many CAPV patients have an enlarged and hypertrophied hepatic artery with a high flow[1,19,50,54] as well as the absence of portal vein, or the presence of hypervascular liver tumor[28]. Such conditions affect the development, function and regenerative capacity of liver, thus predisposing to the development of nodular dysplasia, hepatocarcinoma, or other benign and malignant hepatic tumors[38]. It was reported that abnormal hepatic circulation is one of the etiological factors for hepatocellular hyperplastic nodular lesions[78-80]. Both CAPV and other situations, such as Budd-Chiari syndrome, cause abnormal hepatic flow or peripheral portal venous thrombosis[81,82]. HCC can occur in patients with chronic Budd-Chiari syndrome[82]. Cells in the hyperplastic nodules contain fat deposits[8,83,84], which can be differentiated from other masses.
Marois et al[16] demonstrated that abnormal thin-walled vessels filled in a retrograde fashion from hepatic arteries, may be too weak to burden the changed flow. FNH is an uncommon benign tumor-like lesion of well-circumscribed hyperplastic liver parenchyma, often with central stellate scars. These lesions are hypervascular and can be supplied exclusively with arterial blood[79,85]. FNH, derived from acquired thrombosis, has been reported as well[85]. NRH is due to obstruction or narrowing of portal branches caused by thrombosis or atrophy of areas with severely impaired blood flow, and hypertrophy of areas with a relatively mild impairment of blood flow, leading to nodular formation[80].
Yoshidome et al[86] observed morphological alterations in the liver parenchyma of patients with congenital portocaval shunts, and proposed that morphological changes in the liver of patients with cirrhosis and acquired portocaval shunts as well as HCC may be explained by a common mechanism, namely reduced portal flow. However, circulatory disturbance alone cannot explain the pathogenesis and the underlying unknown mechanism[25]. Simple occlusion of the portal vein and a compensatory increase in arterial blood flow have been proved insufficient for nodule formation[82]. Although rare, fibrosis may develop due to hemodynamic imbalance[20], because flow disturbance only affects hyperplastic hepatic cells but not mesenchymal cells. CAPV occasionally involves HCC, and 40% of HCC patients have no cirrhosis or chronic liver disease[87,88], suggesting that HCC is related to genetic alterations[38]. Indeed, early genetic alterations or the common genetic pathways of hepatic tumors and CAPV would allow accurate comprehension of the commensalisms.
Intrahepatic bile ductules develop from the primitive ductal plate. It was reported that the portal vein plays a crucial role in the formation and remodelling of the ductal plate[89]. Lack of remodeling of the ductal plates results in persistence of an excess of embryonic bile duct structures. This is why biliary atresia and choledochal cysts concur in CAPV patients.
PSE
When the portal vein is absent, toxic metabolites such as ammonia and bile acids collected from the gastrointestinal tract have to bypass the liver directly drainage into the systemic circulation, thus may initiate hepatic encephalopathy. Interestingly, PSE is rarely observed in CAPV patients with mild hyperammonemia and CAPV patients show no clinical manifestations of hepatic encephalopathy until they become obvious[41]. Only a small number of CAPV cases present with subclinical PSE[23,90-92]. Although PSE is not usually observed in CAPV patients, the serum ammonia level in such patients is not always highly elevated. In fact, a significantly low blood ammonia level in SMV is discovered in patients with CAPV[36,37], suggesting that this low level might indicate the presence of a homeostatic control mechanism[37]. The presence of compensatory alterations in intestinal bacterial flora has been suggested as an explanation[9,15,16]. Kamiya[36] analyzed intestinal flora in faeces of CAPV patients and healthy persons before operation, and did not isolate any microorganisms with a strong urease activity. However, intestinal microorganisms isolated from the faeces of patients after operation produced as much urease in vitro as from healthy volunteers[36], indicating that some inhibitory factors for urease-positive microorganisms may exist in the intestinal tract. It was reported that lactulose is effective on hyperammonemia of hepatic encephalopathy by inducing a remarkable growth of Lactobacillus to produce lactic acids which interfere with urease-producing microorganisms[93,94]. It is also possible that proteolysis might be inhibited in the intestine of patients, causing decreased production of ammonia[36]. Kavukcu et al[95] hold the opposite opinion as among the varieties of bacteria displaying urease activity, only three species have been detected: Klebsiella pneumonia, Enterococcus avium, and Peptostreptococcus productus. Moreover, the number of these bacteria is extremely small with no significant differences observed in the flora in fecal specimens obtained before, during, and after surgery[95]. Another likely explanation is that PSE might be due to the increased sensitivity of an aging brain to ammonia and other toxic materials[23] or that homeostatic control may gradually become disordered with increasing age[52]. The brain sensitivity to ammonia or other toxic metabolites may increase with aging[96]. Healthy brain may tolerate to high ammonia levels, while aging brain may not cope with high ammonia and other metabolites and develop symptoms[96-98]. Such mechanisms may contribute to the delayed presentation with hyperammonemia-related encephalopathy[99]. Moreover, another plausible one is that the thin anastomoses at birth slowly become large as the patient ages. The shunt ratio may play a certain role in the occurrence of symptoms[100]. Certain special unknown mechanisms underlying CAPV lead to the delayed PSE.
Others
Bile acids are synthesized in the liver, secreted into bile ducts, and expelled into the intestinal lumen where they are reabsorbed into the systemic circulation via the lymphatic system followed by hepatic uptake from the portal vein, and then metabolized in the liver. However, when the portal vein is absent, these fatty acids, after absorption by the intestinal tract, assume the form of chylomicrons that are transported into the vena cava. Before arriving at the liver, blood from the vena cava reaches the capillaries of peripheral tissues, including adipose and muscular tissue where fat is accumulated, and this presumably constitutes the cause of obesity in such cases[37]. Gitzelmann et al[67] detected patients with hypergalactosaemia along with congenital portosystemic shunts[66-68], and proposed that high blood galactose found in newborns is useful for detecting this abnormality. Hypergalactosaemia might also result from insulin resistance (IR) which is correlated to LD[47]. Imbalance between vasodilator and vasoconstrictor substances has been reported in CAPV patients with HPS[27,30,60-63], and decrease in metabolism or synthesis of these substances in the liver is responsible for the imbalance[60]. Mehrotra et al[101] observed extrahepatic portal vein obstruction in children with high serum levels of growth hormone and somatostatin (IGF-I), and showed that there is some resistance to the action of growth hormones[101]. Chronic anemia (secondary to loss of blood caused by bleeding and/or hypersplenism), and intestinal venous congestion with secondary malabsorption may interfere with the growth rate[31]. Liver dysfunction causing hepatotrophic hormone deprivation also results in growth retardation[40,59,102]. Takeichi[34] reported a CAPV case of patient with chronic renal failure in a vicious circle of toxic materials, leading to severe encephalopathy with coma, waiting for liver transplantation (LT).
However, since CAPV cases have individual series of presentations, more or less, subtle or obvious, the pathology of these manifestations seems to be complicated and covered. Further study is needed to confirm its etiology by analyzing more entities.
IMAGEOLOGY
Imaging abnormality is often coincidentally discovered in children with portal vein disorder. Routine liver examination can reveal the extraordinary mass in majority of these children, triggering further imaging exploration. Portosystemic shunt diagnosis is usually based on clinicopathologic and portographic findings[103]. Portography, ultrasonography, scintigraphy, CT and magnetic imagining are used in diagnosis of this portal vein disorder.
Doppler ultrasonography has been extensively used in evaluating vessels of the abdomen. It is most useful for determining flow direction and pattern besides liver tumor. However, the most important advantage of this technique is its noninvasiveness and no requirement for anesthesia. It yields much useful information about the detailed vascular anatomy as well as hemodynamics[104]. Prenatal screening and intraoperative ultrasonography is especially superior to other techniques[104]. Although color Doppler sonography first depicted the image of the absent portal vein in most cases, ultrasonography (US) may fail to accurately detect the associated extrahepatic shunts because of its subtle US features[31]. Experience and good ultrasound system, knowledge of the examination protocol, and familiarity about the ultrasound anatomy of abdominal vessels and portal vein abnormalities, contribute to the accurate diagnosis of CAPV.
Accurate depiction of intra and extrahepatic vascular anatomy will undoubtedly guide management decisions and surgical or angiographic approaches[21]. Cross-sectional imaging (CT and MR) is very helpful in depicting the course of portosystemic shunt and in identifying absent vessels and type of malformations[42,50]. Currently, 3D-computed tomography angiography (3D-CTA) and magnetic resonance angiography (MRA) can confirm CAPV and visualize the portosystemic shunt[50]. It was reported that multi-slice CTA displays even small vascular branches and has superior spatial resolution to MRA[105]. The posterior or short gastric veins cannot be visualized in patients with portal vein disorders by conventional angiographic portography, but can be clearly revealed by 3D-CT portography[106]. However, a breath-holding technique has been recommended to prevent motion artifacts during scanning by Tsuji et al[8] who assumed that if a patient is able to hold the breath, 3D-CTA can easily capture the entire abnormal vasculature during one breath holding. Unfortunately, CAPV predominately occurs in children difficult to hold breath that urges us to search the substitute. MRA is also a reliable and noninvasive diagnostic modality for the portal venous system[49]. MR imaging can be used both in diagnosis of CAPV and in evaluation of focal hepatic lesions[41]. In addition, previous reports indicate that the presence of a portosystemic shunt may cause lesions of middle cerebellar peduncles, which are responsible for cerebellar symptoms[91]. MR imaging can be used to find cerebral lesions. It was recently reported that conventional high-resolution MR angiography seems unnecessary[41], as the spatial resolution time of MR angiography is almost equivalent. Since 3D-MRA obtained from quality multiplanar reformatted images and volume-rendered images has the advantage of high temporal resolution, contamination from overlapping vascular structures can be avoided, flow dynamics can be assessed, and quality feature is obtainable even in young children with free breaths because the technique is relatively insensitive to motion artefacts[41]. Perhaps, it will be widespread in the diagnosis of CAPV several years later.
Although cross-sectional imaging in most cases can approximately suggest the diagnosis of portal vein disorder, the definitive diagnosis can be made only with catheter angiography[15] and by additional histological analysis of the hepatic parenchyma that demonstrates the absence of hepatic portal venules within the portal triad[35]. Mesenteric portovenography, a usually indirect technique depicting the portal system anatomy, can clarify CAPV abnormality and extrahepatic shunts. In recent years, CO2-wedged venography is considered a good and safe technique for demonstrating the portal circulation[107]. It has such advantages over indirect portography obtained during visceral arteriography[108] as only a venous puncture is required, free and wedged pressure measurements can be obtained with no iodinated contrast medium injected, and transvenous liver biopsy can be made at the same time. However, opacification of portal vein branches could not be obtained[35].
Portovenography can facilitate measurement of the pressure gradient indicating vein blood flow and selected embolism shunts as a therapy. In dogs, transvenous retrograde portography[109] is less invasive than operative mesenteric portography and allows measurement of portal pressures before and after temporary shunt ligation. It also helps differentiate rich-vessel tumor and confirm parenchymal magnetic resonance imaging (MRI) findings. Conventional angiography is not good for children, although it is fairly safe[49,110].
Rectal portal scintigraphy plays an important part in suspected abnormalities of portal circulation and is precise to quantitate portosystemic shunts and valuable for clinical diagnosis[111]. During performing this kind of examination, shunt indexes (SI) are calculated, relative portal hemodynamics can be observed noninvasively, and portal collateral circulation can be detected as well[112]. This technique is hopeful to be extensively applied in detecting CAPV if not expensive.
DIFFERENTIAL DIAGNOSIS
Definitive diagnosis of CAPV should exclude many seemingly resemble cases. For example, histologically confirmed absence of portal vein in the liver is mandatory in the diagnosis. Abernethy type II was previously misclassified as CAPV[21]. Kerlan et al[96] reported a case similar to CAPV, but surgery for closing the fistula between portal vein and inferior cava, revealed an intrahepatic portal vein. Absence of stigmata in patients with portal venous hypertension is an important clue to the final diagnosis[40]. Radiologically, absence of the portal vein must be distinguished from portal vein thrombosis[15,28], based on the absence of venous collaterals or other secondary signs of PH, such as splenomegaly or ascites. Compensatory hypertrophy of the hepatic artery may be present[19]. Appel et al[51] assumed that it is a secondary phenomenon, most properly due to thrombotic occlusion of the extrahepatic portal vein. However, gradual thrombosis of the portal vein stem may allow the development of collaterals without acute dramatic episodes, similar to CAPV. The term of portal vein “aplasia” or “agenesis” in such cases is inadequate since intrahepatic bile ducts are normal[51,113]. Extrinsic compression of tumors, such as HCC and extrahepatic malignant tumor, especially pancreatic adenocarcinoma[5], is another reason. If the portal flow is ceased, initial thrombus arises asymptomatically, the only sign may be the formation of new vessels, which on Doppler US is known as “portal cavernoma” or “cavernomatous transformation” due to the blood volume at the site[102]. It is not easy to differentiate this condition from Abernethy. It was reported that hepatic nodules with rich artery blood flow may prevent influx of portal blood resulting in increased sinusoidal pressure, the portal vein will not be visualized at portography[82]. Owing to the progressive growth of tumor in the omentum and mesentery, increased portal flow would produce extrahepatic portosystemic venous shunts[114].
During fetal life, ductus venosus is the continuation of umbilical vein, which directly inflows into the inferior vena cava, allowing blood returning through the umbilical vein to bypass the portal venous system[115,116]. Failure to close patent ductus venosus within 2 wk after birth would lead to portosystemic encephalopathy or malformations, including congenital heart disease and minor abnormalities[117]. Hepatic nodular lesions, such as FNH, and PSE, have been reported in patients with patent ductus venosus[117,118]. Although the clinical manifestations of patent ductus venosus and CAPV are similar, the mechanism is different. The treatment of CAPV needs liver transplantation and surgical ligation of the patent ductus venosus[18].
It is difficult to differentiate hepatic benign lesions (FNH, FNH-like lesions, and NRH) from malignant lesions (HCC or hepatoblastoma) by imaging except in cases of such abnormal hepatic circulation while transvenous liver biopsy is a valuable alternative diagnostic tool[35,119]. Intrahepatic portal vasculature abnormalities should be differentiated from congenital diseases, such as idiopathic non-cirrhotic PH, portal venous hypoplasia, or hepatic microvascular dysplasia. Histologic findings of these liver diseases are similar to those seen in congenital liver diseases. Thus, it is not possible to differentiate these diseases from congenital shunts[120]. CAPV with PSE should be differentiated from mental disorder involving hyperammonemia or galactosemia due to metabolic deficiencies[121].
Potential etiologies of extrahepatic shunts include shunt formation in association with PH and mesenteric adhesion due to prior abdominal surgery, abdominal trauma, and congenital shunts[97,98,122]. As a result, congenital extrahepatic portosystemic shunts in CAPV patients must be confirmed by excluding the existence of the three liver diseases.
TREATMENT
Treatment options for extrahepatic portosystemic venous shunts strongly depend on the type of Abernethy abnormalities (Figure 2). Balloon-occluded retrograde transvenous obliteration (BRTO), embolization with metallic coils and surgical correction of the shunts are available[97]. In type II patients previously diagnosed with CAPV, occlusion of the shunt is indicated in case of serious symptoms such as hepatic encephalopathy[4] or lateral bleeding. The occlusion techniques include surgical ligation[56,96,123] and interventional embolization[124-126]. Otake et al[99] used coils as embolic materials, because they can progressively occlude the shunt, avoiding acute overload of the portal venous system, and confirmed that there is no evidence that the shunt vessels were recanalized after a two-year follow-up period. The treatment for venous shunts in type I patients without severe symptoms other than liver tumors and LDs is inactive, indicating that close clinical, biochemical, and imaging follow-up should be performed.
The treatment for such patients depends on the conditions of liver neoplasm, such as size and histology[20,50]. It involves LT for hepatoblastoma[26] and chemotherapy for hepatoblastoma after resection of the right hepatic lobe[16]. The choice of treatment for liver tumor in CAPV patients is radical resection of the tumor[39], although mostly it is benign, because the mass becomes larger and then progresses to malignancy[8,26]. Morse et al[28] reported a patient with CAPV who finally underwent LT for hepatoblastoma initially diagnosed as FNH 2 years ago[26,28], suggesting that long-term follow-up and monitoring for malignancy are mandatory, even for benign tumors.
After tumor resection, liver regeneration is said to be dependent on hepatotropic factors in the portal venous blood[37,73]. However, in the patients resected for liver tumor reported[12,15,16,24,37], the resection was uncomplicated and the postoperative course uneventful, despite the absence of gut-derived hepatotropic factors to stimulate liver regeneration. Stimuli other than those transported with the portal blood stream must thus be sufficient to ensure an adequate postoperative liver regeneration in these patients[39].
Some authors hold that increased blood flow comes mainly from the SV in patients with PH and hypersplenism or megalosplenia, indicating that partial splenic embolization can decrease the blood flow and pressure of the main portal vein, similar to the conjoint effects of splenectomy and devascularization[127]. Surgical decompression is also recommended for selected children in order to promote their growth[128].
CAPV has been thought to be asymptomatic and has no indication for LT, and only a few cases having been reported[2,17,27,56,129]. More and more surgeons hold that LT is necessary when medical therapy cannot relieve CAPV-associated abnormalities, such as CAPV- associated cirrhosis caused by biliary atresia[4,17], diffuse hepatoblastoma involving both lobes of the liver[26], and severe portosystemic encephalopathy[56]. No surgical method is available for reconstructing the portal structures of the native liver[56,59]. Shinkai et al[56] reported that LT is an effective surgical treatment for symptomatic CAPV patients when the disease is unresponsive to medical treatment, and believe that prophylactic LT is justified for patients with CAPV before the development of fatal pulmonary complications, such as pulmonary hypertension or HPS, which might complicate or preclude LT[56]. Woodle et al[17] successfully transplanted liver for a biliary atresia patient, and assumed that CAPV is not a contraindication for LT. Taoube et al[130] performed the first paediatric liver transplant for a patient with portal venous agenesia, using the de piggy-back technique.
Recently, auxiliary partial orthotopic liver transplantation (APOLT) was developed in order to reverse fulminant hepatic failure (FHF), which is advantageous over the orthotopic liver transplantation (OLT) and avoids eliminating regeneration of the native liver and a life-long immune suppression[131]. It also has been utilized as an aid in small-for-size grafts to larger recipients during living donor liver transplantation (LDLT)[131]. Soejima et al[27] performed LT for a male patient using a left lateral segment graft from his mother to preserve his native right lobe. Configuration of the donor PV, hepatic artery, HV and bile duct was normal. The results indicate that APOLT is an ideal procedure for patients with CAPV[27]. However, APOLT has certain drawbacks, such as portal steal phenomenon and potential risk of developing tumors in the remnant native liver.
Both the anatomy of portal vein and the function of liver can be restored, and other liver dysfunctions-associated complications may be relieved after LT, such as disappearance of high-intensity lesions in the brain[59].
In conclusion, the prognosis of CAPV patients depends on congenital heart disease, liver disease, and the site of portosystemic shunts. The outcome of CAPV patients with no other abnormalities is different. A long-term follow-up including laboratory tests and image screening is recommended for CAPV patients[20,35,50,126].