Published online Oct 7, 2008. doi: 10.3748/wjg.14.5723
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: October 7, 2008
AIM: To compare the recovery of thrombocytopenia and splenomegaly during long-term follow-up after liver transplantation in patients receiving a living donor transplant or a cadaveric donor transplant.
METHODS: This was a retrospective cohort study of 216 consecutive liver transplant patients who survived for > 6 mo after transplantation; 169 received a liver transplant from a living donor and 47 from a cadaveric donor. The platelet counts or spleen volumes were examined before transplant, 1, 6, and 12 mo after transplant, and then annually until 5 years after transplant.
RESULTS: The mean follow-up period was 49 mo (range, 21-66). Platelet counts increased continuously for 5 years after orthotopic liver transplant. The restoration of platelet counts after transplant was significantly slower in patients with severe pretransplant thrombocytopenia (< 50 000/μL) until 4 years after transplant (P = 0.005). Donor type did not significantly affect the recovery of platelet count and spleen volume in either patient group. In multivariate analysis, pretransplant severe thrombocytopenia (< 50 000/μL) was an independent factor associated with sustained thrombocytopenia (P < 0.001, odds ratio 6.314; confidence interval, 2.828-14.095). Thrombocytopenia reappeared after transplant in seven patients with portal flow disturbance near the anastomosis site.
CONCLUSION: Our study suggests that severe thrombocytopenia before transplant is closely associated with delayed recovery of platelet count after transplant and donor type did not affect the recovery of thrombocytopenia. The reappearance of thrombocytopenia after transplant should be considered a possible indicator of flow disturbance in the portal vein.
- Citation: Chang JH, Choi JY, Woo HY, Kwon JH, You CR, Bae SH, Yoon SK, Choi MG, Chung IS, Kim DG. Severe thrombocytopenia before liver transplantation is associated with delayed recovery of thrombocytopenia regardless of donor type. World J Gastroenterol 2008; 14(37): 5723-5729
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5723.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5723
| Characteristics (n = 216) | Data |
| Age at transplant (range), yr | 49.1 ± 7.7 (21-66) |
| Duration posttransplant (range), mo | 49.1 ± 29.5 (6-178) |
| Male/Female (%) | 158/58 (73/27) |
| Donor type (%) | |
| LDLT | 169 (78) |
| CDLT | 47 (22) |
| Indication for transplant (%) | |
| Hepatitis B cirrhosis | 113 (52.3) |
| Hepatitis C cirrhosis | 3 (1.4) |
| Alcoholic cirrhosis | 8 (3.7) |
| Mixed type cirrhosis1 | 6 (2.8) |
| Cryptogenic cirrhosis | 3 (1.4) |
| Hepatocellular carcinoma2 | 75 (34.7) |
| Autoimmune hepatitis | 5 (2.3) |
| Wilson’s disease | 1 (0.5) |
| Primary biliary cirrhosis | 1 (0.5) |
| Child-Pugh score | 9.5 ± 2.2 (5-14) |
| Cirrhosis complication3 (%) | |
| Ascites | 130 (60) |
| Variceal bleeding | 53 (25) |
| Encephalopathy | 81 (38) |
| SBP | 24 (11) |
| Hepato-renal syndrome | 4 (2) |
| Platelet count, pretransplant (× 103/μL) | 58 ± 36 (9-340) |
| Platelet group (%) | |
| Group 1 (< 30 000/μL) | 29 (13) |
| Group 2 (30 000-50 000/μL) | 80 (37) |
| Gourp 3 (50 000-100 000/μL) | 88 (41) |
| Group 4 (≥ 100 000/μL) | 19 (9) |
| Spleen volume, pretransplant (mm3, n = 193) | 1105 ± 636 (128-4858) |
| Sustained thrombocytopenia1 | Univariate analysis | Multivariate analysis | |||
| Absent (n = 144) | Present (n = 56) | P | P | OR (95% CI) | |
| Age at transplant (yr) | 48.8 ± 7.9 | 48.6 ± 7.3 | ns | ||
| Gender | ns | ||||
| Male | 108 | 40 | |||
| Female | 36 | 16 | |||
| Cirrhosis complication, pretransplant | ns | ||||
| Present | 110 | 44 | |||
| Absent | 34 | 12 | |||
| MELD, pretransplant (LDLT) | ns | ||||
| > 25 | 20 | 6 | |||
| ≤ 25 | 91 | 38 | |||
| Child classification, preptranplant | ns | ||||
| C | 75 | 34 | |||
| A and B | 69 | 22 | |||
| Platelet count, pretransplanta | < 0.001 | < 0.001 | 6.314 (2.828-14.095) | ||
| Group 1 and 2 | 56 | 45 | |||
| Group 3 and 4 | 88 | 11 | |||
| Spleen volume, pretransplanta | < 0.001 | 0.004 | 8.464 (2.001-35.810) | ||
| > 2000 mm | 3 | 10 | |||
| ≤ 2000 mm3 | 124 | 41 | |||
| Donor type | ns | ||||
| LDLT | 111 | 44 | |||
| CDLT | 33 | 12 | |||
| Acute rejection | ns | ||||
| Present | 21 | 5 | |||
| Absent | 123 | 51 | |||
| Mycophenolate mofetil use | ns | ||||
| Yes | 44 | 13 | |||
| No | 100 | 43 | |||
| Biliary complication, posttransplant | ns | ||||
| Present | 36 | 16 | |||
| Absent | 108 | 40 | |||
| Portal flow disturbance, posttransplant | ns | ||||
| Present | 3 | 4 | |||
| Absent | 141 | 52 | |||
Thrombocytopenia develops commonly in the early postoperative period after liver transplantation[1-3], and most instances of early thrombocytopenia recover with restoration of hepatic function[4,5]. By contrast, the recovery of pretransplant long-lasting thrombocytopenia differs among patients, and some patients experience persistent thrombocytopenia even several years after the operation[6]. Persistent thrombocytopenia increases the risk of bleeding-related complications, which worsen the prognosis for the transplanted patients[1,7,8].
The possible causes of thrombocytopenia soon after the operation include consumption of platelets in the graft liver, impairment of platelet synthesis, small graft, and sepsis[1,3,7-10]. However, the causes or contributing factors of persistent thrombocytopenia (< 100 000/μL) beyond one year after the operation are not well known. A previous report suggested that thrombocytopenia at 3 and 6 mo after transplantation is an independent contributing factor to persistent thrombocytopenia[11].
Patients receiving a living donor transplant restore the liver volume up to 80% of the whole liver, in contrast with cadaveric donor transplant. Because of this deficient restoration in liver volume, the posttransplant recovery of thrombocytopenia or splenomegaly in living donor transplant is assumed to be different than that in cadaveric donor transplant[10].
The aims of this study were to compare the recovery of thrombocytopenia and splenomegaly in patients receiving a living donor liver transplantation (LDLT) and cadaveric donor liver transplantation (CDLT), and to identify the factors contributing to persistent thrombocytopenia during long-term follow-up after orthotopic liver transplantation (OLT).
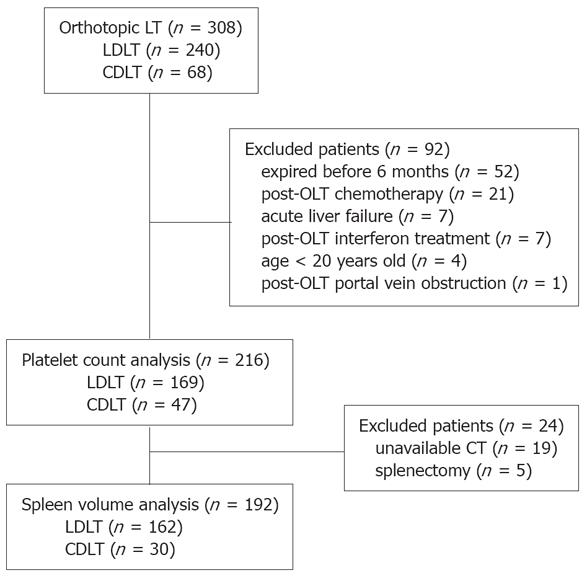
We performed 289 OLTs in our transplantation center between July 1996 and June 2006. Among them, 49 patients had liver transplant from cadaveric donor and 240 patients from living donor who donated right lobe. In addition, 19 patients prepared their transplants with us and then had CDLT performed in China during the same period. They were sent to us soon after transplants and followed up. Ninety-two patients were excluded from this study because of death within 6 mo after OLT (n = 52), post-OLT chemotherapy (n = 21), acute liver failure (n = 7), post-OLT interferon treatment (n = 7), age less than 20 years old (n = 4), and post-OLT portal vein obstruction (n = 1). The remaining 216 patients (169 LDLT, 47 CDLT) were recruited consecutively (Figure 1). Patient’s anonymity was preserved and the study protocol conforms to the ethical standards of the responsible committee on human experimentation and with the Helsinki declaration of 1975, as revised in 1983.
The patients comprised 158 men and 58 women with a mean age at transplant of 49.1 ± 7.7 years (range, 21-66 years) (Table 1). The indications for OLT included complications of advanced cirrhosis in 133 patients, hepatocellular carcinoma in 75 patients, and other causes in seven patients. The causes of liver diseases were hepatitis B virus in 188 patients, hepatitis C virus in 9 patients, alcoholic liver diseases in 13 patients, autoimmune diseases in 5 patients, and other causes in 2 patients. The follow-up duration after the operation ranged from 6 to 178 mo, with a mean of 49 mo.
Serial platelet counts were obtained from the medical record before the OLT and 1, 6, 12, 24, 36, 48, and 60 mo after the OLT. Pretransplant thrombocytopenia was classified arbitrarily according to platelet count as follows: group 1 (< 30 000/μL), group 2 (30 000-50 000/μL), group 3 (50 000-100 000/μL), group 4 (≥ 100 000/μL), which was modified according to the WHO adverse event criteria for hematologic toxicity. To evaluate spleen volume, the greatest length, transverse diameter, and thickness at the hilum of the spleen were measured using abdominal computed scanning tomography before the OLT and 1, 6, 12, 24 and 36 mo after the OLT. These values were multiplied together and then by the factor of 0.6 to obtain an estimated spleen volume as described previously[12]. Sustained thrombocytopenia was defined as platelets count < 100 000/μL 12 mo after the OLT. Factors such as demographic and clinical features associated with sustained thrombocytopenia were also assessed.
The immunosuppressive regimen used comprised cyclosporine or tacrolimus combined with or without mycophenolate mofetil, which was withdrawn in patients with severe leucopenia (< 2 000/μL). In addition, corticosteroid was used for 3-6 mo after operation, and then it was tapered. Among 216 patients, 132 (61%) patients had taken cyclosporine, 130 (60%) patients had tacrolimus, and 127 (59%) patients had mycophenolate mofetil. Mycophenolate mofetil was used for short duration, less than 6 mo, in most patients.
Rejection was diagnosed by liver biopsy, which showed Banff rejection activity index more than 3. Patients who had endoscopic retrograde biliary procedures or percutaneous transhepatic biliary procedures performed were diagnosed as those with biliary complication. We defined patients as those with portal flow disturbance if there was clear evidence in abdominal computerized tomography or magnetic resonance imaging.
Continuous variables are expressed as mean ± SD unless stated otherwise. Factors associated with sustained thrombocytopenia were analyzed by univariate analysis. Significant factors by univariate analysis (P < 0.05) were subjected to multivariate analysis using logistic regression (forward selection). Continuous variables were compared by paired t test and repeated-measures analysis of variance (ANOVA), and categorical variables were compared by either the chi-square test or Fisher exact test, as appropriate. The statistical analyses were performed using SPSS for Windows version 14 (Chicago: SPSS Inc., USA). P < 0.05 was considered significant.
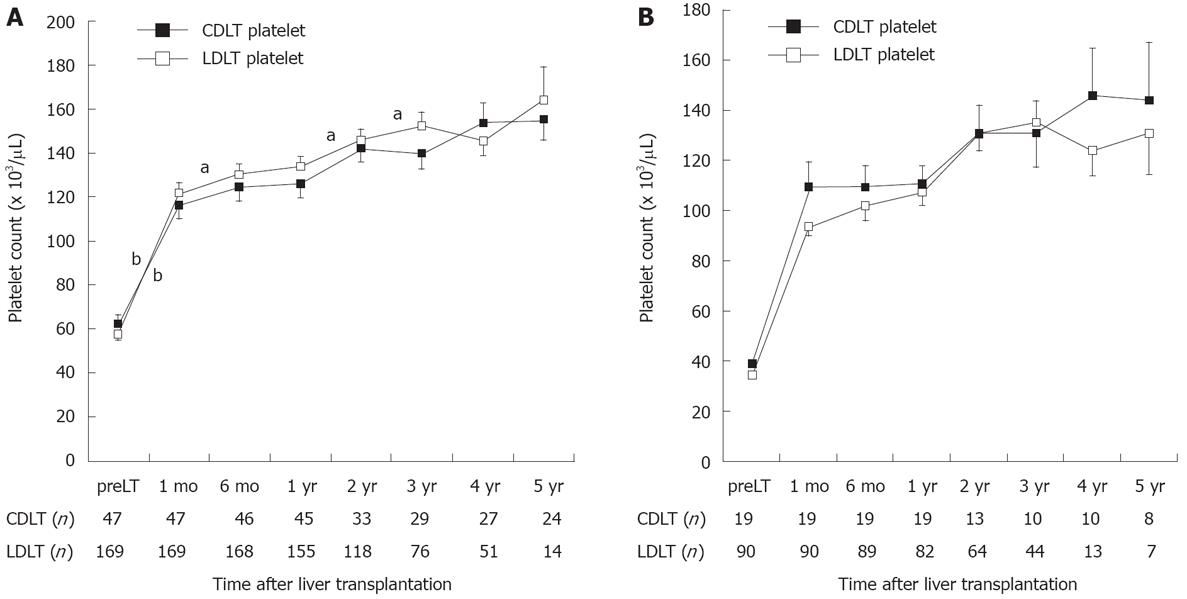
Ninety-one percent (197/216) of our studied population demonstrated evidence of moderate to severe thrombocytopenia, as indicated by a platelet count < 100 000/μL before the OLT. The percentages of patients according to platelet count before the operation were 13% in group 1 (< 30 000 μL), 37% in group 2 (30 000-50 000/μL), 41% in group 3 (50 000-100 000/μL), and 19% in group 4 (≥ 100 000/μL). The mean platelet counts increased continuously for 5 years after orthotopic liver transplant: 58 000/μL before OLT, 121 000/μL at 1 mo, 128 000/μL at 6 mo, 132 000/μL at 12 mo, 145 000/μL at 24 mo, 148 000/μL at 36 mo, 149 000/μL at 48 mo, and 158 000/μL at 60 mo after OLT. The platelet counts increased significantly during the first month after OLT compared with later periods (P < 0.001), and then increased gradually until 5 years after the operation (Figure 2A). The donor type did not affect the recovery of platelet count after the operation and the recovery of platelet count in patients with severe thrombocytopenia before the operation (groups 1 and 2) was also not different between LDLT and CDLT (Figure 2B).
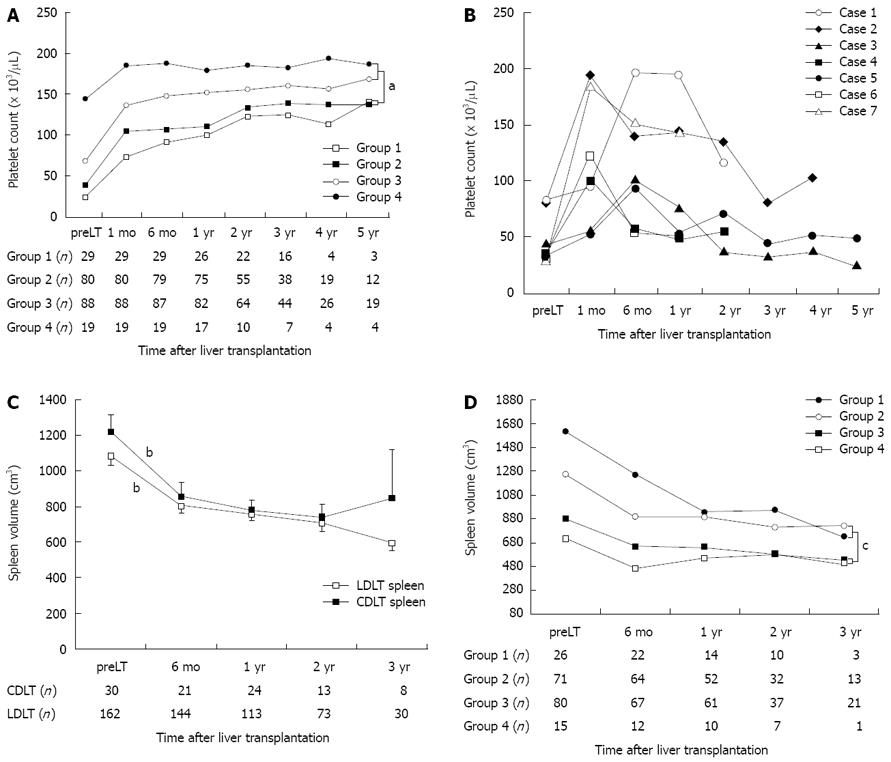
Recovery of platelet count after OLT differed significantly between the four groups classified according to pretransplant thrombocytopenia until 5 years after the OLT (P = 0.015, repeated-measures ANOVA) (Figure 3A). In those who survived more than 1 year, 58% (15/26) of patients in group 1, 30% (30/75) in group 2, 12% (10/82) in group 3, and 6% (1/17) in group 4 had moderate to severe thrombocytopenia (< 100 000/μL) one year after the OLT. Five years after the OLT, the mean platelet count in patients with pretransplant severe thrombocytopenia (groups 1 and 2) was < 150 000/μL. In contrast, patients with pretransplant moderate thrombocytopenia (group 3) showed a faster recovery of platelet count to a mean value near 150 000/μL (148 000/μL) 6 mo after the OLT. The restoration of platelet counts after the OLT was significantly slower in patients with severe pretransplant thrombocytopenia (groups 1 and 2) than in the groups with mild or moderate thrombocytopenia (groups 3 and 4) until 4 years after the OLT (P = 0.005, Figure 3A).
In univariate analysis, clinical factors associated with sustained thrombocytopenia (< 100 000/μL at 12 mo after OLT) were pretransplant severe thrombocytopenia (< 50 000/μL) and pretransplant large spleen volume (> 2000 mm3) (P < 0.001, respectively; Table 2). In the multivariate analysis, pretransplant severe thrombocytopenia and splenomegaly were independent factors associated with sustained thrombocytopenia (P < 0.001 and P = 0.004, respectively).
Seven patients with portal vein thrombosis or stenosis showed a decrease in platelet count in proportion to the progression of portal flow disturbance. The disturbance of portal flow appeared between 6 mo and 1 year after the operation. In these patients, the platelet count increased initially as in the other patients but decreased gradually with disturbed portal flow (Figure 3B). The causes of portal flow disturbance were attributed to main portal vein thrombosis (three patients) and stenosis of the portal vein anastomosis site (four patients). The donor types for these patients were three cadaveric donors and four living donors.
All patients with splenomegaly showed decreased spleen volume 6 mo after the operation. The mean volumes of spleens were 1105 ± 636 cm3 before transplant, 810 ± 469 mm3 6 mo after OLT, 761 ± 385 mm3 12 mo after OLT, 711 ± 399 mm3 24 mo after OLT, and 648 ± 415 mm3 36 mo after OLT, and it did not return to normal until 3 years. The reduction rate of spleen volume relative to the pretransplant volume was 26.7% and 31.1% 6 and 12 mo after the operation, respectively. The speed of volume reduction was fastest within the first 6 mo after the operation compared with later periods up to 2 years (P≤ 0.02; Figure 3C). The rate of reduction in spleen volume did not differ significantly between the LDLT and CDLT groups. The restoration of spleen volume after the OLT was significantly slower in patients who had severe pre-OLT thrombocytopenia (groups 1 and 2) than in those with mild to moderate thrombocytopenia (groups 3 and 4) until 2 years after the operation (P = 0.006, repeated-measures ANOVA; Figure 3D).
This long-term cohort study clearly demonstrated that liver transplantation affects the rate of recovery of thrombocytopenia. The recovery was fastest within the first month and then decreased gradually for 5 years. Most previous reports have shown the impact of whole liver transplantation from cadaveric donor on hypersplenism during the early postoperative period[13-16]. Recent reports showed that partial liver transplantation from living donor effectively reduces spleen size and resolves thrombocytopenia in adults and children, but the follow-up duration was shorter than in the present study[17-19]. Compared with CDLT, LDLT could not restore the whole liver volume. This suggests that the recovery rate of thrombocytopenia after the operation may differ between patients receiving LDLT and CDLT. However, our study included a large number of LDLT patients, showed that LDLT effectively restored platelet count at a rate similar to that observed in CDLT patients. This indicates that LDLT rapidly resolved the portal hypertension despite the partial restoration of the liver up to 80% of the whole liver volume, and regeneration of a living donor liver is as effective as regeneration after CDLT.
The causes of thrombocytopenia during the early postoperative period include sequestration of platelets in the liver graft, reduced platelet production, immunological reaction, and graft dysfunction[1,3,7-9]. However, the causes of sustained long-term thrombocytopenia for more than 1 year are not fully understood. A previous study suggested that pretransplant variceal bleeding, pretransplant splenomegaly, and thrombocytopenia at 3 and 6 mo after LT are predicting factors of persistent thrombocytopenia[11]. In our study, pretransplant severe thrombocytopenia (< 50 000/μL) and splenomegaly (> 2000 mm3) were significantly associated with sustained thrombocytopenia. In addition, delayed graft failure, biliary complication, and infections are considered other possible causes of sustained thrombocytopenia lasting more than 1 year. In our study, most biliary complication induced transient thrombocytopenia rather than sustained thrombocytopenia. Almost these patients with biliary complication recovered from thrombocytopenia after biliary intervention.
Splenomegaly is sustained for several years after the operation even though the graft liver restores normal portal hemodynamics relatively soon after the operation[16]. In a prospective hemodynamic study, hemodynamic parameters including cardiac index, mean arterial pressure, portal flow velocity, and hepatic artery resistance index improved within 6 mo after liver transplantation[15]. However, the spleen size decreased gradually beyond 2 years. Our study is consistent with the results of previous studies in showing that the long-lasting severe splenomegaly takes several years to resolve although liver synthetic function recovers rapidly.
In seven of our patients, thrombocytopenia reappeared after the initial recovery of platelet counts because of disturbed portal flow. The possibility of portal vein complications is considered greater after LDLT than after CDLT[20,21]. Vascular complications in pediatric patients with whole liver grafts and segmental grafts in a large series of 600 transplants was reported[20]. The incidence of portal vein complications was higher in LDLT patients (27%) than in patients receiving reduced-size or split-liver transplantation (1%) or whole liver transplantation (1%) from a cadaveric donor. Portal vein thrombosis or stenosis appearing in immediate postoperative period should be treated with surgical treatment or radiological intervention[22-25]. If the stenosis or thrombosis progresses slowly beyond 1 year, as in our patients, the patients may not have any symptoms because of reopening of the previously established collateral circulation. Thus, the clinician should monitor the recovery of platelet count after the operation carefully. If thrombocytopenia reappears after the initial recovery of platelet count, the degree of portal vein stenosis or collateral circulation should be evaluated by dynamic computed tomography. In addition, biliary tract infection or interferon therapy for recurrent hepatitis C may cause the reappearance of thrombocytopenia[26,27].
Our study has some limitations. First, it is a retrospective cohort study. However, a large number of patients (216 patients) were included consecutively in the cohort and the follow-up duration was longer than that of other studies. Second, we did not perform hemodynamic studies to evaluate the resolution of portal hypertension after the operation and did not measure serum thrombopoietin level to evaluate the synthetic function of the platelet[28-30]. Further study including hemodynamic analysis is needed to identify why the patients with severe pretransplant thrombocytopenia improve slowly after the operation.
In conclusion, this study demonstrated that severe thrombocytopenia before transplantation is closely associated with delayed recovery of platelet count after transplantation. Our data also showed that hypersplenism improve at the same rate after LDLT as after CDLT. If thrombocytopenia reappears beyond 6-12 mo without any other cause, disturbance of portal flow, especially in the anastomosis site of the portal vein, should be evaluated.
The recovery of pretransplant long-lasting thrombocytopenia differs among patients. Because of the deficient restoration in liver volume, the posttransplant recovery of thrombocytopenia or splenomegaly in living donor transplant is assumed to be different from that in cadaveric donor transplant.
The study has not been done to compare the recovery of thrombocytopenia and splenomegaly in patients receiving a living donor liver transplantation and cadaveric donor liver transplantation during long-term follow-up after orthotopic liver transplantation.
This study suggests at the first that severe thrombocytopenia before transplant is closely associated with delayed recovery of platelet count and spleen volume after transplant, and donor type does not affect the recovery of thrombocytopenia.
The degree of thrombocytopenia before transplant can be used to predict delayed recovery of platelet count and spleen volume after transplant.
This is a retrospective study comparing recovery of thrombocytopenia and splenomegaly in patients receiving a LDLT and CDLT. The manuscript is generally well written and has a concise, simple methodology with good long-term follow-up.
Peer reviewer: Adam G Testro, PhD, Department of Gastro-enterology and Liver Transplantation, Austin Health Institution, Heidelberg 3032, Australia
S- Editor Li DL L- Editor Li M E- Editor Ma WH
| 1. | McCaughan GW, Herkes R, Powers B, Rickard K, Gallagher ND, Thompson JF, Sheil AG. Thrombocytopenia post liver transplantation. Correlations with pre-operative platelet count, blood transfusion requirements, allograft function and outcome. J Hepatol. 1992;16:16-22. |
| 2. | Randoux O, Gambiez L, Navarro F, Declerck N, Labalette M, Dessaint JP, Pruvot FR. Post-liver transplantation thrombocytopenia: a persistent immunologic sequestration? Transplant Proc. 1995;27:1710-1711. |
| 3. | Richards EM, Alexander GJ, Calne RY, Baglin TP. Thrombocytopenia following liver transplantation is associated with platelet consumption and thrombin generation. Br J Haematol. 1997;98:315-321. |
| 4. | Chatzipetrou MA, Tsaroucha AK, Weppler D, Pappas PA, Kenyon NS, Nery JR, Khan MF, Kato T, Pinna AD, O'Brien C. Thrombocytopenia after liver transplantation. Transplantation. 1999;67:702-706. |
| 5. | Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: friend or foe? Liver Transpl. 2008;14:923-931. |
| 6. | Iglesias-Berengue J, Lopez-Espinosa JA, Ortega-Lopez J, Sanchez-Sanchez L, Asensio-Llorente M, Margarit-Creixell C, Diaz-Heredia C. Hematologic abnormalities in liver-transplanted children during medium- to long-term follow-up. Transplant Proc. 2003;35:1904-1906. |
| 7. | Plevak DJ, Halma GA, Forstrom LA, Dewanjee MK, O'Connor MK, Moore SB, Krom RA, Rettke SR. Thrombo-cytopenia after liver transplantation. Transplant Proc. 1988;20:630-633. |
| 8. | Munoz SJ, Carabasi AR, Moritz MJ, Jarrell BE, Maddrey WC. Postoperative thrombocytopenia in liver transplant recipients: prognostic implications and treatment with high dose of gamma-globulin. Transplant Proc. 1989;21:3545-3546. |
| 9. | Miyata T, Yokoyama I, Todo S, Tzakis A, Selby R, Starzl TE. Endotoxaemia, pulmonary complications, and thrombo-cytopenia in liver transplantation. Lancet. 1989;2:189-191. |
| 10. | Marubashi S, Dono K, Miyamoto A, Takeda Y, Nagano H, Umeshita K, Monden M. Impact of graft size on postoperative thrombocytopenia in living donor liver transplant. Arch Surg. 2007;142:1054-1058. |
| 11. | Sutedja DS, Wai CT, Teoh KF, Lee HL, DaCosta M, Kaur M, Lee YM, Lee KH, Mak K, Quak SH. Persistent thrombocytopenia in liver transplant patients. Transplant Proc. 2004;36:2331-2333. |
| 12. | Shah SH, Hayes PC, Allan PL, Nicoll J, Finlayson ND. Measurement of spleen size and its relation to hypersplenism and portal hemodynamics in portal hypertension due to hepatic cirrhosis. Am J Gastroenterol. 1996;91:2580-2583. |
| 13. | Yanaga K, Tzakis AG, Shimada M, Campbell WE, Marsh JW, Stieber AC, Makowka L, Todo S, Gordon RD, Iwatsuki S. Reversal of hypersplenism following orthotopic liver transplantation. Ann Surg. 1989;210:180-183. |
| 14. | Soresi M, Bascone F, Magliarisi C, Campagna P, Di Giovanni G, Riili A, Carroccio A, Montalto G. Hemodynamic changes in splanchnic circulation after orthotopic liver transplantation in patients with liver cirrhosis. Abdom Imaging. 2002;27:541-545. |
| 15. | Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58-64. |
| 16. | Witte M, Langnas AN, Hirst K, Stratta RJ, Shaw BW Jr. Impact of liver transplantation on the reversal of hypersplenism. Transplant Proc. 1993;25:1987. |
| 17. | Kaneko J, Sugawara Y, Akamatsu N, Kokudo N, Makuuchi M. Spleen volume and platelet number changes after living donor liver transplantation in adults. Hepatogastroenterology. 2004;51:262-263. |
| 18. | Egami S, Sugawara Y, Mizuta K, Kaneko J, Kawarasaki H, Makuuchi M. Effect of pediatric living-donor liver transplantation on splenomegaly. Transplantation. 2002;74:1639-1642. |
| 19. | Kim SH, Lee JM, Choi JY, Suh KS, Yi NJ, Han JK, Choi BI. Changes of portosystemic collaterals and splenic volume on CT after liver transplantation and factors influencing those changes. AJR Am J Roentgenol. 2008;191:W8-W16. |
| 20. | Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Kelly S, Brady L, Leef JA, Millis JM. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658-666. |
| 21. | Broniszczak D, Szymczak M, Kaminski A, Chyzynska A, Ismail H, Drewniak T, Nachulewicz P, Markiewicz M, Teisseyre J, Dzik E. Vascular complications after pediatric liver transplantation from the living donors. Transplant Proc. 2006;38:1456-1458. |
| 22. | Koo BY, Yu HC, So MC, Jin GY, Kwak HS, Cho BH. Endovascular management of early portal vein thrombosis caused by coronary vein steal after liver transplantation and its outcome. Clin Transplant. 2008;22:668-671. |
| 23. | Lendoire J, Raffin G, Cejas N, Duek F, Barros Schelotto P, Trigo P, Quarin C, Garay V, Imventarza O. Liver transplantation in adult patients with portal vein thrombosis: risk factors, management and outcome. HPB (Oxford). 2007;9:352-356. |
| 24. | Yerdel MA, Gunson B, Mirza D, Karayalcin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. |
| 25. | Ko GY, Sung KB, Lee S, Yoon HK, Kim KR, Kim KM, Lee YJ. Stent placement for the treatment of portal vein stenosis or occlusion in pediatric liver transplant recipients. J Vasc Interv Radiol. 2007;18:1215-1221. |
| 26. | Iacob S, Gheorghe L, Hrehoret D, Becheanu G, Herlea V, Popescu I. Pegylated interferon alpha-2a and ribavirin combination therapy in HCV liver transplant recipients. Experience of 7 cases. J Gastrointestin Liver Dis. 2008;17:165-172. |
| 27. | Ross AS, Bhan AK, Pascual M, Thiim M, Benedict Cosimi A, Chung RT. Pegylated interferon alpha-2b plus ribavirin in the treatment of post-liver transplant recurrent hepatitis C. Clin Transplant. 2004;18:166-173. |
| 28. | Peck-Radosavljevic M, Wichlas M, Zacherl J, Stiegler G, Stohlawetz P, Fuchsjager M, Kreil A, Metz-Schimmerl S, Panzer S, Steininger R. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95:795-801. |
| 29. | Peck-Radosavljevic M, Zacherl J, Wichlas M, Sims P, Meng YG, Panzer S, Lipinski E, Steininger R, Muhlbacher F, Pidlich J. Thrombopoietic cytokines and reversal of thrombocytopenia after liver transplantation. Eur J Gastroenterol Hepatol. 1999;11:151-156. |
| 30. | Chang FY, Singh N, Gayowski T, Wagener MM, Mietzner SM, Stout JE, Marino IR. Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation. 2000;69:70-75. |