Published online Oct 7, 2008. doi: 10.3748/wjg.14.5689
Revised: September 16, 2008
Accepted: September 23, 2008
Published online: October 7, 2008
AIM: To evaluate the accuracy of automated blood cell counters for ascitic polymorphonuclear (PMN) determination for: (1) diagnosis, (2) efficacy of the ongoing antibiotic therapy, and (3) resolution of spontaneous bacterial peritonitis (SBP).
METHODS: One hundred and twelve ascitic fluid samples were collected from 52 consecutive cirrhotic patients, 16 of them with SBP. The agreement between the manual and the automated method for PMN count was assessed. The sensitivity/specificity and the positive/negative predictive value of the automated blood cell counter were also calculated by considering the manual method as the “gold standard”.
RESULTS: The mean ± SD of the difference between manual and automated measurements was 7.8 ± 58 cells/mm3, while the limits of agreement were +124 cells/mm3 [95% confidence interval (CI): +145 to +103] and -108 cells/mm3 (95% CI: -87 to -129). The automated cell counter had a sensitivity of 100% and a specificity of 97.7% in diagnosing SBP, and a sensitivity of 91% and a specificity of 100% for the efficacy of the ongoing antibiotic therapy. The two methods showed a complete agreement for the resolution of infection.
CONCLUSION: Automated cell counters not only have a good diagnostic accuracy, but are also very effective in monitoring the antibiotic treatment in patients with SBP. Because of their quicker performance, they should replace the manual counting for PMN determination in the ascitic fluid of patients with SBP.
- Citation: Riggio O, Angeloni S, Parente A, Leboffe C, Pinto G, Aronne T, Merli M. Accuracy of the automated cell counters for management of spontaneous bacterial peritonitis. World J Gastroenterol 2008; 14(37): 5689-5694
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5689.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5689
| Variables | Cirrhotic patients with SBP |
| n | 16 |
| Sex (M/F) | 11/5 |
| Age (yr) | 63.8 ± 10.3 |
| Child-Pugh class (A/B/C) | 0/8/8 |
| Alcoholic origin (No/Yes) | 10/6 |
| Treatment efficacy at 48 h (No/Yes) | 11/5 |
| Resolution of infection (No/Yes) | 12/4 |
Spontaneous bacterial peritonitis (SBP) is a well-recognized and potentially fatal complication in cirrhotic patients with ascites[1-3]. The prevalence of SBP in hospitalized patients has been reported to range between 10% and 30%[4-6]. The mortality rate related to this complication remains high, approximately 20%, despite the recent improvements achieved in the management of this complication[7-9]. A rapid diagnosis and a prompt treatment are essential for the survival of these patients. SBP symptoms, however, are not always present and may be insidious; in addition, the ascitic fluid cultures require several days to grow and, in the clinical practice, they are negative in more than 60% of patients with SBP[10].
For these reasons, the current guidelines[10] recommend the use of polymorphonuclear (PMN) cell count in the ascitic fluid for diagnosing SBP, suggesting that a PMN cell count greater than 250 cells/mm3 should be considered highly suspicious for SBP, thus providing an indication to empirically initiate the antibiotic treatment.
To date, PMN cell count is routinely performed by using the traditional hematological method with a light microscope in a manual counting chamber (Burker chamber). The manual laboratory counting of ascitic PMN is however laborious, time-consuming, and costly. Moreover, it is not always timely available in all hospitals, especially in those small patient care units with limited laboratory facilities, and it cannot be frequently performed on an emergency basis (at night or on weekends). The manual system, therefore, too often delays the initiation of the important sequence of events that should lead to a rapid diagnosis and treatment of this infection.
In the last years, a series of reports proposed the use of urinary reagent strips to achieve an “instant” bedside diagnosis of SBP[11-14] with promising results[15-17]. A recent prospective multicenter study[18] in which 2123 paracenteses were performed in 1041 patients, however, reports the lack of any diagnostic efficacy of the strip-test. The authors concluded that a routine cytological examination remains mandatory for the diagnosis of SBP. Moreover, the urine screening test has never demonstrated to be useful in the monitoring of PMN cell count at follow-up paracenteses performed 48 h after the beginning of the treatment for SBP. According to current guidelines[10], in fact, the antibiotic treatment empirically administered should be changed if a decrease in the PMN count of less than 25% of the pre-treatment value is not obtained. A qualitative method providing only a negative/trace/positive score needs therefore to be always confirmed by standard cytology of the ascitic fluid.
A valid alternative to manual PNM counting is represented by automated blood cell counters, commonly and largely used in all laboratories for blood cell counting; they offer accurate and rapid differential counts of leukocytes, there including PMN. Our previous study[19], published in 2003, demonstrated that automated blood cell counters are a reliable tool for the rapid diagnosis of SBP and our experience was also confirmed by Cereto et al[20]. Moreover, automated cell counters, as a quantitative method able to provide a reliable PMN value not requiring further confirmation, could be useful not only for diagnostic purposes, but also for determining the effectiveness of the ongoing empiric antibiotic therapy.
The aim of the present study was therefore to evaluate the validity of the automated blood cell counter not only for SBP diagnosis, but also for monitoring the responsiveness to the ongoing antibiotic treatment. For this purpose, we compared the determination of PMN count in the ascitic fluid obtained by the manual and the automated methods at basal and follow-up diagnostic paracenteses in a group of cirrhotic patients with SBP.
A total of 112 ascitic fluid samples was collected from 52 consecutive cirrhotic patients with ascites (36 men and 16 women, mean age 65.3 ± 11.7 years) hospitalized at our Gastroenterology Unit. The diagnosis of liver cirrhosis was based on clinical, biochemical, and/or histopathological data. The severity of the liver disease was classified in each patient at entry, according to the Child-Pugh[21] scores.
All the patients underwent a routine abdominal paracentesis at the time of hospitalization. Paracentesis was repeated if, during hospitalization, the patient had signs or symptoms compatible with infection (i.e. fever, change in the mental status, abdominal pain, peripheral leukocytosis, development of renal failure, hypotension, etc.). In some patients re-admitted for recurrent ascites, a diagnostic paracentesis was also repeated. Two samples of ascitic fluid for each patient were collected under aseptic conditions in tubes containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant. White blood cells (WBC) and PMN counts were determined by both the traditional method with a light microscope in a manual counting chamber, and the automated cell blood counter (Technicon System H*1; Bayer Diagnostics, Milan, Italy), as previously described[19]. The specimens were analyzed within 1 h. Additional samples of ascitic fluid were collected for the determination of albumin and total protein concentrations. Moreover, 10 mL of ascitic fluid were directly inoculated at the patient’s bedside into aerobic and anaerobic blood culture bottles for bacteriological examination[22].
For traditional manual WBC and PMN counts, ascitic fluids were collected in tubes containing 0.084 mL of 15% EDTA. Ten milliliters were centrifuged at 1500 r/min for 10 min; 9 mL of the supernatant were discharged and 40 μL of the remaining ascitic fluid were diluted with 800 μL of Turk’s fluid and gently shaked; 20 μL were used to fill the counting chamber. The cells were counted (× 40) in one of the nine large squares, and the number of WBC per cubic millimeter was calculated. Another sample of 10 mL of ascitic fluid was used for the PMN percentage determination (× 100), after centrifugation and May-Grünwald-Giemsa staining.
For WBC and PMN cell counts by the automated method, 100 μL of ascitic fluid, collected in tubes containing 0.054 mL of 15% EDTA anticoagulant, were directly injected into the analyzer.
SBP was diagnosed when the PMN cell count in the ascitic fluid was greater than 250 cells/mm3 and an antibiotic treatment with i.v. cefotaxime (2 g/8 h, for a minimum of 5 d) was empirically initiated in all the patients with these values, regardless of the positivity of the culture. The antibiotic dosage was adjusted to the renal function throughout the treatment period and the efficacy was evaluated by further diagnostic paracenteses 2 and 5 d after the beginning of the treatment. A further paracentesis was performed in the patients with no resolution at 5 d.
In those cases not responding to the initial antibiotic regimen, the therapy was appropriately changed, either according to the in vitro susceptibility of the isolated bacteria, or empirically. For this purpose, a further paracentesis was always performed 2 d after the beginning of the antibiotic treatment. Treatment failure was established when the condition of the patients rapidly deteriorated within the first hours of the antibiotic therapy (i.e. with development of shock), or when no significant decrease in the ascitic PMN count was observed in the follow-up paracentesis. A reduction in the PMN count of less than 25% of the pre-treatment value was considered as suggestive of failure of the antibiotic treatment[10].
At the time of the 48-h paracentesis, as well as at the following paracentesis, WBC and PMN counts were performed by both manual method and automated cell counter.
SBP was considered resolved when PMN count in the ascitic fluid had decreased to less than 250 cells/mm3.
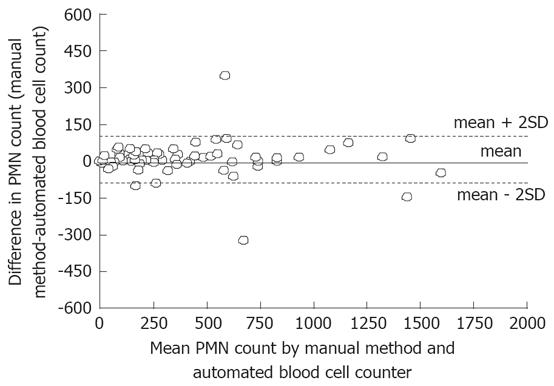
Results are expressed as mean ± SD. The values of PMN count determined by the two methods were compared using the Student’s t test. The agreement between the two techniques was assessed by using the method suggested by Bland and Altman[23]. The differences between the results of the manual counting and the automated blood cell counter in each patient were plotted against the mean of the two readings observed in each patient. The mean and SD of the differences were calculated. The limits of agreement, defined as the mean ± 2 SDs of the difference, and their 95% confidence intervals (CI) were then calculated.
By considering the PMN count determined by the traditional manual method as the “gold standard”, the sensitivity/specificity and the positive/negative predictive value of the automated blood cell counter were calculated according to Ransohoff and Feinstein[24] for the following end-points: (1) diagnosis of SBP defined as a PMN count of more than 250 cells/mm3; (2) treatment efficacy defined as a decrease in the PMN count of more than 25% of the pre-treatment value at the 48-h diagnostic paracentesis; (3) resolution of the infection defined as a reduction of PMN count to less than 250 cells/mm3.
The statistical significance was established at a P < 0.05. Calculations were performed by using a statistical software program (Number Cruncher Statistical System 97).
A total of 112 samples of ascitic fluid were collected from 52 consecutive cirrhotic patients (36 male/16 female; age: 65.3 ± 11.7 years; Child-Pugh class: 24 B/28 C; alcoholic origin: 29%) with ascites. The degree of agreement between the measurements of PMN count in the ascitic fluid, using the manual method or the automated blood cell counter, is reported in Figure 1. The mean ± SD of the difference between the manual and the automated measurements was 7.8 ± 58 cells/mm3, while the limits of agreement were +124 cells/mm3 (95% CI: +145 to +103) and -108 cells/mm3 (95% CI: -129 to -87).
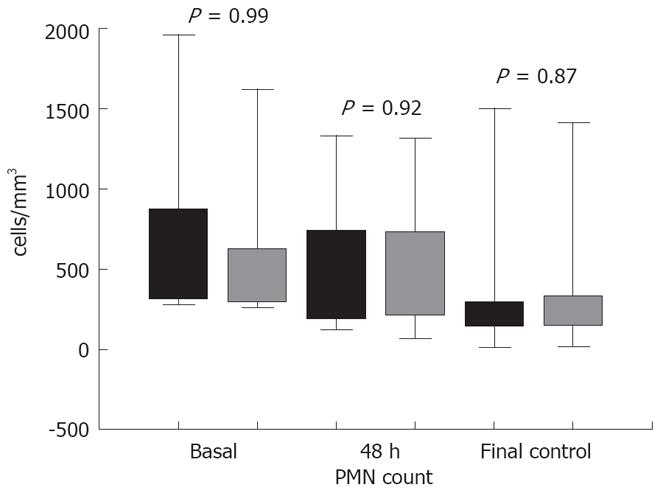
SBP, as indicated by a PMN count > 250 cells/mm3 with the traditional manual method, was diagnosed in 16 patients. Demographic, clinical characteristics, and outcome of these 16 patients with SBP are reported in Table 1. No significant differences were observed when PMN counts were determined by using both methods (Figure 2).
As far as the diagnosis of SBP is concerned, the agreement between the two methods was observed in all the patients but one, who had a PMN count of 270 cells/mm3 at the automated blood cell counter and of 249 cells/mm3 at the manual method (false positive result). By considering a PMN count > 250 cells/mm3 determined by the manual method as the “gold standard” for the SBP diagnosis, the automated blood cell counter had a sensitivity of 100% and a specificity of 97.7%, whereas positive and negative predictive values were 94.1% and 100%, respectively.
As far as the efficacy of the antibiotic treatment is concerned, the agreement between the two methods was obtained in all the patients but one, in whom the therapy was not considered effective by the automated counter only (false negative result). By considering the treatment efficacy as a reduction in the PMN count > 25% of the pre-treatment value (determined by the traditional manual method) as the “gold standard”, the automated blood cell counter had a sensitivity of 91% and a specificity of 100%, whereas positive and negative predictive values were 100% and 83.3%, respectively.
By considering the resolution of infection as a reduction of PMN count to less than 250 cells/mm3 (determined by the traditional manual method) at the final paracentesis as the “gold standard”, the automated blood cell counter showed a complete agreement (sensitivity 100%, specificity 100%, positive and negative predictive values 100%).
In the guidelines for the diagnosis and treatment of SBP in cirrhotic patients[10] published in the year 2000, the International Ascites Club suggested that a PMN cell count greater than 250 cells/mm3 should be considered highly suspicious for SBP, thus providing an indication to promptly initiate an empiric antibiotic treatment. Forty eight hours after the initiation of antibiotics, a repeat diagnostic paracentesis is also recommended, either to document the response by a greater-than-25% decrease in the ascitic fluid neutrophil count, or to induce a change in the antibiotic therapy. A further diagnostic paracentesis showing that PMN cell count is below 250 cells/mm3 is finally indicated to confirm the infection resolution and stop the antibiotic treatment.
To date, PMN cell count is routinely performed by using the traditional hematological method with a light microscope in a manual counting chamber. Urinary reagent strips have been used to make an “instant” bedside diagnosis of SBP with controversial results[12,18]. This screening test, however, is a qualitative method providing only a negative/trace/positive score; it is therefore, by definition, unable to monitor the PMN cell count at follow-up paracentesis (performed 48 h after the beginning of the treatment). In the management of SBP, the cytological examination remains then mandatory. Manual laboratory counting of ascitic PMN is however laborious, time-consuming, costly, and not always timely available in all the hospitals. Automated blood cell counters, commonly and largely used in laboratories for blood cell counting, offer instead an accurate and rapid count of PMN in the ascitic fluid and has proven to be a reliable tool for the rapid diagnosis of SBP[19,20]. This finding is confirmed by the present study in which we analyzed with both methods 112 samples of ascitic fluid collected from 52 consecutive cirrhotic patients with ascites. Although the agreement limits (as reported in Figure 1) may in fact range between +124 and -108 cells/mm3, SBP was correctly diagnosed by automated blood cell counter in all the cases but one (a false positive result). The use of the automated method as the only diagnostic tool, therefore, would have erroneously submitted this patient to an antibiotic treatment: a less significant error than that of no treating a patient who actually needed to be treated.
However, in order to suggest the use of automated blood cell counters as an alternative to manual counting, its usefulness for the optimization of the antibiotic treatment should be demonstrated. The agreement between the two methods should therefore be obtained not only in the first diagnostic paracentesis, but also in those performed for monitoring the antibiotic treatment. The paracentesis performed after 48 h is in fact particularly significant, since the efficacy of the antibiotic treatment is usually established in this phase. As a matter of fact, in case of SBP, in the large majority of the patients antibiotics are chosen empirically-that is, without the support of the result of the ascitic fluid culture. This is because antibiotics should be started immediately after the result of the PMN cell count[10] (before the result of the culture), and because the ascitic fluid culture outcome-by using conventional culture techniques-may be negative in up to 60% of patients with SBP. The reduction of more than 25% of the initial PMN cell count value is the criterion to establish the efficacy of the antibiotic treatment. The present study showed that, at the diagnostic paracentesis performed 48 h after the start of the antibiotic treatment, the two methods agreed in all the patients but one, in whom a false negative result was obtained. By using the automated cell counter, this patient would then be erroneously considered as a non-responder to the ongoing antibiotic treatment. This would have led to a switch in the antibiotic treatment, a less significant mistake than that of erroneously considering the patient as a responder to the ongoing antibiotic treatment. As far as the infection resolution assessed at the final diagnostic paracentesis is concerned (based on a PMN count below 250 cell/mm3), an agreement between the two methods was achieved in all the patients. These results suggest that automated cell counters should be considered a reliable tool not only for the diagnosis of SBP, but also in its optimal management; automated methods could therefore definitely replace manual counting. The benefits of a quicker and precise method in the evaluation of the ascitic fluid have been clearly stressed[25].
Another, although less important, advantage of the automated cell counting method over both reagent strips and the manual method is the possibility to precisely assess the amount of PMN in a bloody (for a traumatic tap or a condition inducing bleeding) ascitic fluid. With the last two methods, the amount of PMN deriving directly from the blood spilled over into the ascitic fluid cannot be differentiated from the amount of PMN due to the infection. A correction factor of 1 PMN per 250 red blood cells has been proposed[10], since this is the maximum expected ratio of PMN to red cell normally present in the peripheral blood. With automated cell counters, a measure of the amount of red blood cells and PMN in both the peripheral blood and the ascitic fluid can be simultaneously obtained. The amount of ascitic PMN due to the infection can be therefore calculated by the real PMN-to-red blood cells ratio in the blood and by the real erythrocytes and PMN number in the ascitic fluid. The simultaneous count of PMN and erythrocytes in the same sample of ascitic fluid cannot be made by manual count, since to obtain a reliable PMN count in a manual counting chamber, erythrocytes should be previously hemolyzed by an acidic solution.
In conclusion, the manual and the automated methods have a good agreement in the determination of PMN in the ascitic fluid. Automated cell counters have a good diagnostic accuracy, not only for the diagnosis, but also for the monitoring of the antibiotic treatment in patients with SBP. Automated cell counters, which offer an easier and quicker PMN count, should therefore replace the manual counting for PMN determination in the ascitic fluid analysis.
Spontaneous bacterial peritonitis (SBP), a severe complication of ascites, is managed on the results of polymorphonuclear (PMN) cell count in the ascitic fluid, which are used both to test the efficacy of the ongoing antibiotic treatment and to establish the resolution of infection.
Manual PMN counting is time-consuming, costly, and not always timely available. Automated blood cell counters, commonly used in all laboratories for blood cell counting, may be a valid alternative.
Automated cell counters provide a reliable ascitic PMN count and have a good diagnostic accuracy, not only for the diagnosis, but also for monitoring the effectiveness of the ongoing antibiotic treatment in patients with SBP.
Automated cell counters, which offer an easier and quicker PMN count, should therefore replace the manual counting for PMN determination in the ascitic fluid analysis.
This is a well-written paper. Authors demonstrated that automated cell counters not only have a good diagnostic accuracy, but are also very effective in monitoring the antibiotic treatment in patients with SBP.
Peer reviewer: Takayuki Yamamoto, MD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Li DL L- Editor Megro F E- Editor Lin YP
| 1. | Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358. |
| 2. | Pinzello G, Simonetti RG, Craxi A, Di Piazza S, Spano C, Pagliaro L. Spontaneous bacterial peritonitis: a prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology. 1983;3:545-549. |
| 3. | Almdal TP, Skinhoj P. Spontaneous bacterial peritonitis in cirrhosis. Incidence, diagnosis, and prognosis. Scand J Gastroenterol. 1987;22:295-300. |
| 4. | Llach J, Rimola A, Navasa M, Gines P, Salmeron JM, Gines A, Arroyo V, Rodes J. Incidence and predictive factors of first episode of spontaneous bacterial peritonitis in cirrhosis with ascites: relevance of ascitic fluid protein concentration. Hepatology. 1992;16:724-727. |
| 5. | Gilbert JA, Kamath PS. Spontaneous bacterial peritonitis: an update. Mayo Clin Proc. 1995;70:365-370. |
| 6. | Garcia-Tsao G. Bacterial infections in cirrhosis: treatment and prophylaxis. J Hepatol. 2005;42 Suppl:S85-S92. |
| 7. | Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis--in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96:1232-1236. |
| 8. | Llovet JM, Planas R, Morillas R, Quer JC, Cabre E, Boix J, Humbert P, Guilera M, Domenech E, Bertran X. Short-term prognosis of cirrhotics with spontaneous bacterial peritonitis: multivariate study. Am J Gastroenterol. 1993;88:388-392. |
| 9. | Toledo C, Salmeron JM, Rimola A, Navasa M, Arroyo V, Llach J, Gines A, Gines P, Rodes J. Spontaneous bacterial peritonitis in cirrhosis: predictive factors of infection resolution and survival in patients treated with cefotaxime. Hepatology. 1993;17:251-257. |
| 10. | Rimola A, Garcia-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. |
| 11. | Sapey T, Mena E, Fort E, Laurin C, Kabissa D, Runyon BA, Mendler MH. Rapid diagnosis of spontaneous bacterial peritonitis with leukocyte esterase reagent strips in a European and in an American center. J Gastroenterol Hepatol. 2005;20:187-192. |
| 12. | Castellote J, Lopez C, Gornals J, Tremosa G, Farina ER, Baliellas C, Domingo A, Xiol X. Rapid diagnosis of spontaneous bacterial peritonitis by use of reagent strips. Hepatology. 2003;37:893-896. |
| 13. | Sapey T, Kabissa D, Fort E, Laurin C, Mendler MH. Instant diagnosis of spontaneous bacterial peritonitis using leukocyte esterase reagent strips: Nephur-Test vs. MultistixSG. Liver Int. 2005;25:343-348. |
| 14. | Kim DY, Kim JH, Chon CY, Han KH, Ahn SH, Kim JK, Paik YH, Lee KS, Moon YM. Usefulness of urine strip test in the rapid diagnosis of spontaneous bacterial peritonitis. Liver Int. 2005;25:1197-1201. |
| 15. | Campillo B, Richardet JP, Dupeyron C. Diagnostic value of two reagent strips (Multistix 8 SG and Combur 2 LN) in cirrhotic patients with spontaneous bacterial peritonitis and symptomatic bacterascites. Gastroenterol Clin Biol. 2006;30:446-452. |
| 16. | Rerknimitr R, Rungsangmanoon W, Kongkam P, Kullavanijaya P. Efficacy of leukocyte esterase dipstick test as a rapid test in diagnosis of spontaneous bacterial peritonitis. World J Gastroenterol. 2006;12:7183-7187. |
| 17. | Thevenot T, Cadranel JF, Nguyen-Khac E, Tilmant L, Tiry C, Welty S, Merzoug N. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients by use of two reagent strips. Eur J Gastroenterol Hepatol. 2004;16:579-583. |
| 18. | Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thevenot T, Silvain C, Bureau C, Nouel O, Pilette C, Paupard T, Vanbiervliet G, Oberti F, Davion T, Jouannaud V, Roche B, Bernard PH, Beaulieu S, Danne O, Thabut D, Chagneau-Derrode C, de Ledinghen V, Mathurin P, Pauwels A, Bronowicki JP, Habersetzer F, Abergel A, Audigier JC, Sapey T, Grange JD, Tran A. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275-1281. |
| 19. | Angeloni S, Nicolini G, Merli M, Nicolao F, Pinto G, Aronne T, Attili AF, Riggio O. Validation of automated blood cell counter for the determination of polymorphonuclear cell count in the ascitic fluid of cirrhotic patients with or without spontaneous bacterial peritonitis. Am J Gastroenterol. 2003;98:1844-1848. |
| 20. | Cereto F, Genesca J, Segura R. Validation of automated blood cell counters for the diagnosis of spontaneous bacterial peritonitis. Am J Gastroenterol. 2004;99:1400. |
| 21. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 22. | Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355. |
| 23. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. |
| 24. | Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299:926-930. |
| 25. | Runyon BA. The evolution of ascitic fluid analysis in the diagnosis of spontaneous bacterial peritonitis. Am J Gastroenterol. 2003;98:1675-1677. |