INTRODUCTION
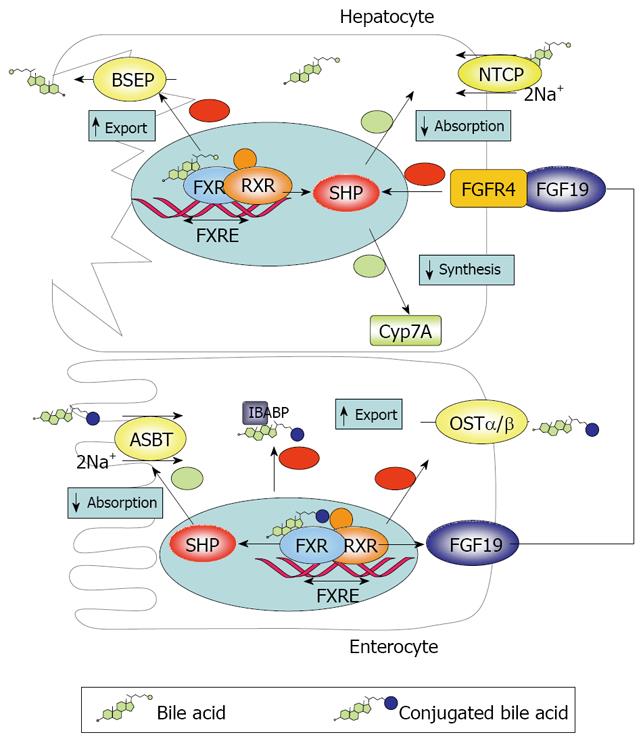
Figure 1 Diagram showing the mechanisms of BA transport and regulation at the intestinal and hepatic level.
Separate chapters in this series of minireviews are devoted to cover various aspects of bile acids (BAs) chemistry, physiology and pathophysiology, including the hepatic synthesis and handling of BAs and their implications in health and disease. Here we will deal with the normal and pathological roles of BAs in one of the traditionally known natural sites of action, i.e. the intestine. It is well known that BAs are secreted into the duodenal lumen after meals in order to act as tensioactives and facilitate fat digestion. This is possible because of the amphipathic characteristics of BAs, which are molecules with a highly hydrophobic core and a number of hydroxyl groups attached. Because these groups may extend to one of the two sides of the basically planar structure formed by the hydrocarbon rings of the BA molecule, its polarity is maximal when all hydroxyl groups are set out in the same side. Thus cholic acid (CA), deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA), all of which have alpha hydroxyl groups, are more efficient tensioactives than ursodeoxycholic acid (UDCA), which has hydroxyls with both alpha and beta conformation. In order to act efficiently as tensioactives BAs must work in coordination with other amphipathic compounds, namely phospholipids, which are another essential component of bile.
Once their physiological function is accomplished, most BA molecules are efficiently reabsorbed in the distal part of the small intestine and reach the liver via portal blood, where they are avidly taken up by hepatocytes. Thus BAs are not wasted but recycled with an almost perfect yield (approximately 95%). The molecular details of this enterohepatic cycle have been elucidated (Figure 1). The type and amount of BAs thus reabsorbed, as well as of those that pass into the colon, are not constant but subject to variation as a result of diet, transit time, drugs, disease, etc. This in turn has an impact on the effects of BAs because they are not equivalent in terms of bioactivity. Certain BAs have been involved in colon cancer, but also in other conditions such as intestinal inflammation, diarrhea, etc.
The dynamics of BAs transport and metabolism is tightly regulated. This is necessary because intracellular accumulation of BAs, as happens in cholestasis, may result in cytotoxicity. Because BAs are synthesized in the liver, an important part of the control of their homeostasis takes place in hepatocytes, the details of these processes are covered in other chapters of this series. However, in part of the regulatory system the intestine plays important direct (transport/metabolism) and indirect (endocrine response of intestinal epithelium to BAs) roles.
BA IN THE INTESTINE
As mentioned above, the liver synthesizes BAs at the expense of cholesterol and also retrieves reabsorbed BAs from the blood. From hepatocytes they are secreted against steep concentration gradients into bile, together with cholesterol and phospholipids. Thus, between meals, most of the pool of BAs resides in the gallbladder ready to be used at short notice. The mechanisms whereby hepatocytes take up BAs from the bloodstream and synthetize and secrete them into bile have been reviewed in other chapters of this series. When food is ingested (more precisely if the meal is rich in fat) the gallbladder contracts in response to cholecystokinin. It has been proposed that intraduodenal BAs exert a negative feedback control on postprandial cholecystokinin release and the resulting gallbladder contraction. Thus the acute (but not chronic) intraduodenal bile salt depletion with cholestyramine affects gallbladder and also antroduodenal motility, possibly by enhanced motilin release[1].
The mix of BAs contained in bile represents a balance of primary and secondary compounds. Primary BAs are those synthesized as such by the liver, and comprise predominantly CA and CDCA. These are secreted to bile mainly conjugated with glycine and taurine, thus having enhanced water solubility. Secondary BAs are derived from primary BAs by modifications carried out by intestinal bacteria. The main modifications are deconjugation, oxidation of hydroxyl groups in 3, 7 and 12 positions, and 7-dehydroxylation[2]. The main secondary BAs are lithocholic acid (LCA) and DCA. The overall result is an increase in the hydrophobicity of BA pool. The transformation of BAs by bacterial enzymes has several important consequences. First, it favors passive absorption in the colon of those BAs escaping the active uptake that takes place in the ileum. If both mechanisms operate normally, only 1%-3% of the amount of BA that is secreted by the liver is ultimately excreted in faeces (deconjugated and otherwise transformed). Second, it increases the potentiality of BAs to cause noxious effect, like carcinogenicity and cholesterol gallstone disease[3]. Third, the composition of the BA pool will vary when the conditions of biotransformation are altered, for instance by changes in transit time or alterations in the microbiota brought about by drugs, diet, etc.[3,4]. For instance, a high fat diet and a long (slowed) transit time favor DCA generation from CA and absorption, which in turn is associated with higher risk of cholesterol gallstones and cancer (see below). In addition, diets rich in fat and poor in fiber can increase more than 10-fold the amount of taurine conjugated BAs reaching the colon, due to higher conjugation and production (higher conjugation reduces ileal absorption)[5,6]. Taurine dietary intake (meat, seafood) also contributes to this result. Overweight and accelerated intestinal transit reduce BA absorption and may cause idiopathic BA malabsorption[7]. Drugs such as cholestiramine bind BAs and reduce absorption.
Owing to the fact that 7-dehydroxylation cannot be reversed by the host enzymatic machinery, LCA and DCA tend to accumulate in the BA pool. However, LCA is 3-sulfated and conjugated at C-24 by the liver, resulting in a derivative that is poorly absorbed from the colonic mucosa, and consequently LCA is not present in significant amounts in the bile[8]. Thus major BAs in human bile are CA, CDCA and DCA, which are accompanied by minor amounts of UDCA, LCA and other BAs, whereas faeces contain mainly DCA, LCA, minor amounts of CDCA, CA and UDCA and a variety of bacteria transformed derivatives[2]. Concentrations of BAs in the intestinal lumen are variable but usually high, estimated in the medium millimolar range. This is consistent with their critical micellar concentration (e.g. 6-10 mmol/L for TCA), i.e. the concentration corresponding to spontaneous formation of micelles[9].
BA TRANSPORT BY EPITHELIAL CELLS
As mentioned above, BAs are efficiently taken up from the lumen of the ileal segment, leaving only approximately 5% (or approximately 0.5 g/d) in the lumen[10]. This fraction is in part passively absorbed in the colon, a process facilitated by bacterial deconjugation, and in part transformed and extruded with faeces. In contrast, ileal uptake is predominantly an active process carried out by the apical sodium-dependent BA transporter (ASBT, gene symbol SLC10A2), which imports BAs coupled to Na+ absorption (1:2 stoichiometry)[11]. ASBT is highly homologous to the hepatocyte Na+/taurocholate cotransporting polypeptide transporter (NTCP, gene symbol SLC10A1), which plays a pivotal role in BA uptake by the liver from the portal bloodstream. BAs species are not equally transported by ASBT. Thus, conjugated (more hydrophilic) BAs are transported more efficiently than unconjugated forms[12,13]. This is physiologically consistent with the fact that deconjugation normally takes place in the colon. The affinity of ASBT is also higher for dihydroxy BAs (CDCA and DCA) than for trihydroxy BAs (CA, taurocholic acid-TCA-, glycocholic acid-GCA-)[12].
BAs are believed to be transferred directly from ASBT to an intracellular 14 kDa protein called ileal BA binding protein (IBABP/FABP6) through the formation of a 2:1 stoichiometric complex[14]. IBABP is supposed to facilitate transport of BAs within the cell to the basolateral membrane. This is suggested by data showing coordinated expression of both ASBT and IBABP in the postnatal development in the intestine and in cholangyocytes, as well as by the fact that IBABP and ASBT form complexes with a defined stoichiometry[15]. It should be noted however that ASBT is expressed in the kidney without being accompanied by IBABP[16], and also that FXR knockout mice, which do not express IBABP, show enhanced rather than inhibited intestinal BA absorption, suggesting that IBABP may function as a negative regulator of intestinal BA reabsorption, at least in the mouse[17]. Interestingly, low IBABP expression has been linked to the risk of necrotizing enterocolitis in an animal model, suggesting that inefficient transfer of BA to the basolateral membrane may ultimately result in epithelial damage and inflammation[18].
Finally, BAs exit the enterocyte via the recently characterized OSTα/β transporter[19], an obligate heterodimer which functions in a Na+-independent manner and also transports prostaglandin E2, estrone-3-sulfate, dehydroepiandrosterone sulfate[13].
Developmentally ileal transport has been described to be preceded by active colonic absorption in rabbits[20]. This differs markedly from adult animals, which show net colonic secretion. The expression of IBABP and ABST is known to be subjected to distinct changes early in life, which are dependent on the species and protein considered. Thus, in rats and mice, ASBT is highly expressed in the ileum of the fetus before birth but is downregulated or entirely absent in the newborn and later upregulated again. IBABP shows a similar ontogenic profile in mice, while in rats it first appears postnatally[20]. The induction of ABST after birth is stimulated by thyroxine in rats[21].
It is interesting to examine the effects of interference with normal transporter function in the intestine. Genetic disruption of ASBT activity or pharmacological inhibition with SC-435 results in BA malabsorption and diarrhea[22-24]. Conversely, Ostα -/- mice show reduced intestinal capacity to take up BAs but unaffected fecal BA output, which is secondary to a marked shrinkage of the BA pool[19]. On the other hand, there are significant numbers of patients with idiopathic ileal BA malabsoprtion who suffer of unexplained chronic diarrhea[25].
The exact role of other transporters is controversial. They may play a minor role in BA handling by the intestine. These include MRP3 and an alternatively spliced form of ASBT, t-ASBT[13].
REGULATION OF INTESTINAL BA TRANSPORT
The intestinal (ileal) absorption of BAs is tightly regulated to meet physiological demands. In addition, intestinal BA uptake has direct and indirect impact on hepatic BA homeostasis. The main factor involved in both functions is the farnesoid X receptor (FXR/NR1H4), which was originally identified as an orphan nuclear receptor that was activated by farnesol, an intermediate in the mevalonate biosynthetic pathway[26]. FXR is expressed in ileal enterocytes and also in the liver, as well as in other tissues, such as the adrenal gland and the kidney[27]. Interestingly, the intestine seems to have the most intense FXR expression in the body[28]. Agonists of the FXR include BAs, particularly CDCA, followed by DCA, LCA and many other BAs with minor efficacy (conjugation does not affect binding)[29]. The α-position of OH groups in BA molecule is very important for interaction with FXR[30]. Upon activation, FXR modulates gene transcription acting in concert with another nuclear receptor, the retinoid X receptor alpha (RXRα), by recognizing a specific promoter sequence called the FXR responsive element. FXR is pivotal in the BA regulation both in the liver and in the intestine. In hepatocytes FXR increases expression of the bile salt export pump (BSEP) and downregulates the expression of NTCP and CYP7A. Since NTCP and BSEP mediate BA uptake from blood and export to bile in hepatocytes and CYP7A catalyzes the limiting-rate step in the classical BA biosynthetic pathway, this leads to reduced BA uptake, decreased synthesis and enhanced export to bile. Thus BA accumulation in hepatocytes tends to be self-limiting. In enterocytes, FXR is coupled to reduced ASBT and increased IBABP and OSTα/β expression, resulting in inhibition of intestinal absorption of BAs and prevention of intracellular BA accumulation.
The repressing effects of FXR are mediated by the transcription factor SHP (Small Heterodimer Partner), which is induced by FXR but lacks a DNA binding domain[31,32]. Instead, SHP binds to other nuclear receptors such as RXR/RAR (retinoic acid receptor), LRH1 (liver receptor homolog 1) and LXR (liver X receptor), inhibiting their transcriptional effects[11,33]. IBABP seems to interact with ASBT and FXR to promote FXR transcription[34].
An important feature of FXR role in regulation of BA homeostasis is that it is not limited to local effects. Rather, sensing of the enterocyte BA pool by FXR affects the liver by way of the endocrine factor FGF19 (Fgf15 in mice)[32,33]. FGF19 is released to the portal circulation and activates fibroblast growth factor receptor 4 (FGFR4) in hepatocytes, which results in downregulation of CYP7A1 and therefore inhibition of the classical BA synthetic pathway, both by SHP induction and possibly other pathways[33]. Thus BAs modulate their own synthesis both by local hepatic and remote intestinal negative feedback. Tissue specific FXR gene knockdown experiments suggest that both pathways are similarly important[27]. The importance of the latter pathway is exemplified by the fact that administration of TCA downregulates CYP7A1 in the liver only when administered intraduodenally, but not after intravenous or portal instillation[35]. However, alternative pathways for feedback control have been proposed[33].
Physiologically, lack of BA uptake by ASBT inhibition leads to increased fecal BA (and diarrhea) and reduced FXR stimulation, lower FGF19 synthesis, and consequently enhanced BA synthesis, expanding the BA pool and lowering plasma cholesterol[24]. In contrast, Ostα -/- mice do not exhibit increased fecal BA output and have downregulated Cyp7a1 expression and a reduced BA pool[19]. This is due to increased Fgf15, secondary to FXR activation by “trapped” BAs.
In addition, FXR activation has been claimed to participate in the regulation of bacterial growth within the intestine. This hypothesis is supported by the findings that cholestasis results in bacterial overgrowth in the small intestine and increased translocation, which are counteracted in experimental models by oral BAs[36,37]. The FXR mediated induction by BAs of antibacterial genes such as angiogenin, carbonic anhydrase 12 and inducible nitric oxide synthase may account for this effect[38].
Other nuclear factors are regulated by BAs, including the pregnane X receptor (PXR/NR1I2), the Vitamin D receptor (VDR/NR1I1) and the androstane constitutive receptor (CAR)[33]. Thus LCA binds and activates intestinal and systemic VDR[39]. It has been proposed that the effects brought about by LCA are essentially local, directed to induce CYP3A genes and to aid in detoxification[40], but at any rate it can substitute for Vitamin D systemically. PXR and CAR activation leads to the upregulation of secondary BA transporters such as MRP2, MRP3 and MDR1. For instance, CA induces MRP2 and MRP3 in the intestine[41]. In addition, BAs have been reported to activate a G-protein associate receptor, named after the fact G protein-coupled BA receptor 1 (also known as TGR5), which is expressed in many tissues including the gastrointestinal tract[42].
IBABP is also regulated by PPARα/β in humans but not mice[43], and can also be indirectly up-regulated by cholesterol through the activation of sterol-responsive element-binding protein 1c (SREBP1c) by LXR[44]. ASBT is also regulated by PPARα[45]. These changes are expected to increase BA uptake and possibly reduce cholesterol absorption, a putative mechanism of action of the hypolipemic drugs fibrates[43]. Corticoids are also known to upregulate ASBT expression[46]. Because BA ileal uptake is inhibited in intestinal inflammation and probably contributes to diarrhea, corticoid treatment may be specifically useful in this setting.
Among the pathological conditions affecting BA homeostasis, cholestasis downregulates ASBT expression[47,48]. The mechanism is unclear, but it may be related to PPARα inhibition by BAs (possibly because of high blood levels), given that PPARα has been reported to transactivate ASBT transcription, as mentioned above[45,49]. Intestinal MRP2 but not MRP3 is decreased by cholestasis in rats[50] and in humans[48,51], although the significance of these findings is uncertain. In contrast, increased absorption has been reported in primary biliary cirrhosis, thus contributing to cholestasis in this condition[52]. Hypertriglicerydemia also reduces ASBT expression and inhibits BA absorption[53], an effect which in turn might exacerbate hypertriglyceridemia[54]. Interestingly, gallstones have been associated with lower intestinal ASBT and IBABP expression in normal weight but not overweight women[55]. These changes are accounted for by lower hepatic FXR and thereby increased BA synthesis.
BA AND COLORECTAL CANCER
There is wide epidemiological evidence linking BA exposure (for instance due to high fat diet) and gastrointestinal (specially colorectal) cancer[56-58]. Patients with colorectal adenomas and carcinomas exhibit high blood and fecal levels of secondary BAs[59,60]. Diets rich in fat are powerful stimulants of BA secretion, as mentioned above. Thus many investigators have studied the effects of BAs, particularly secondary BAs (DCA and LCA) on intestinal epithelial cell proliferation, apoptosis and mutagenesis in vitro, as well as on cancer promotion in vivo. Paradoxically DCA, but not CA or UDCA, exhibit proapoptotic effects on cell lines, which appear to depend on a variety of mechanisms[61,62]. The ability of BAs to induce apoptosis has been linked to their hydrophobicity, so that unconjugated DCA and CDCA are the most powerful inducers[63]. This makes sense, since only hydrophobic BAs can gain access to colonic cells via passive diffusion.
Different mechanisms have been involved in the proapoptotic effect of BAs. Direct increase in mitochondrial membrane permeability has been suggested, leading to mitochondrial swelling, release of cytochrome c and apoptosis[64]. Alterations in plasma membrane composition with subsequent up-regulation of caveolin-1 may underlie also the activation of protein kinase C by BAs[65]. DCA and UDCA have opposing effects on PKC translocation, affecting a number of isoenzymes including PKC alpha, epsilon and beta1[66,67]. DCA also activates NF-κB and AP-1 in colonic epithelial cells, downstream of PKC stimulation[68]. As expected, UDCA has the opposite effect. ERK activation has been involved in DCA proapoptotic effects, inasmuch as genetic or pharmacological inhibition blocks them[69]. In addition, DCA (and CDCA) induce c-Fos and COX2 in intestinal epithelial cells[70].
Despite these observations, it is important to note that epithelial cells may develop resistance to DCA induced apoptosis, which is achieved partly via the NO pathway[71] and is correlated with shifted expression of multiple proteins, as assessed by proteomic analysis[72]. Alternatively, additional factors may protect against DCA induced apoptosis, such as glutathione-S-transferase P1-1[73]. The expected result would be the selection of transformed cells, favoring the formation of adenomas and predisposing to subsequent development of cancer.
BAs also exert direct actions that can lead to tumorigenesis. Thus, DCA has genotoxic effects, which are believed to be secondary to induction of oxidative stress in the cell[74], and suppresses the p53 response to DNA damage, an action that is at least partly dependent on ERK signaling[75]. Moreover, inhibition of BRCA-1 by relatively high DCA concentrations contributes to defective DNA repair[76]. Recently DCA and LCA (in conjugated form) were shown to elicit transactivation of the epidermal growth factor receptor via interaction with muscarinic receptors and phosphorylation of ERK[77]. Another gene target of DCA via ERK is the tumor marker EphA2 receptor protein tyrosine kinase[78]. In general, these actions are not shared by UDCA and may be opposed by it[61,79,80]. Taken together, these data indicate that DCA may behave as a co-carcinogenic and/or cancer promoter agent, which may potentiate the activity of any primary carcinogen or cancer initiator. In addition, DCA may increase tumor invasiveness by activation of beta-catenin signaling[81]. An interesting observation is that FXR expression is diminished in colon cancer[82]. Under these circumstances, FXR-mediated mechanisms involved in the prevention of BA accumulation in these cells could be expected to be completely or partly inactive, thus exacerbating BA-induced effects.
We also count on substantial in vivo evidence about the effects of BAs on colorectal cancer. Thus colonic grafts from mice with an APC gene mutation do not develop adenomas if they are removed from the fecal stream[83]. In the standard Min mouse model UDCA produces a dose dependent decrease in the number of intestinal tumors, showing synergism with the cycloxygenase 2 inhibitor sulindac[84]. In the azoxymethane model of cancer associated to chronic colitis, UDCA lowered the multiplicity of colonic adenocarcinoma, while sulfasalazine had no significant effect[85]. Similar results were obtained in the regular (without colitis) azoxymethane model[86]. The chemopreventive effect of UDCA is associated with decreased Ras activation and COX2 expression[87].
This type of observations can be extended to human disease. Thus, a study carried out in patients with primary biliary cirrhosis undergoing surveillance by colonoscopy revealed a non-significant reduction in the prevalence of colorectal adenomas and, more importantly, a lower probability of recurrence (7% vs 28% at 3 years, P = 0.04)[88]. UDCA lowers cancer mortality (but not incidence) in ulcerative colitis patients with sclerosing cholangitis[89]. In a clinical trial on the secondary prevention of colorectal cancer, UDCA caused a non-significant 12% decrease in recurrence rate but a significant reduction (39%) in the subgroup with high-grade dysplasia[90]. In clinical trials of cancer associated to inflammatory bowel disease, a condition which increases the risk of developing cancer, UDCA has been shown to be beneficial, ranging from a mild chemoprotective effect[91] to a clear decrease in the relative risk of developing colorectal dysplasia or cancer[92]. Mechanistically, UDCA has been reported to reduce mucosal proliferation in cancer naive patients[88] but to have no effect in adenoma patients[93]. From a pharmacokinetic point of view, the main effect of UDCA administration in humans is an increase of luminal (fecal) UDCA/DCA ratio, although DCA absolute levels remain unaltered[94].
An intriguing possibility is that taurine, which is bound to a substantial fraction of the BA pool, contributes to cancer risk. Taurine, which can be released in the intestinal lumen from conjugated BAs due to the metabolic activity of several bacterial strains, is metabolized by the intestinal flora yielding hydrogen sulfite, which increases colonocyte turnover and inhibits butyrate metabolism. Although these cells oxidize efficiently this compound to thiosulfate, taurine derived hydrogen sulfite may be involved in carcinogenesis. In fact, defects in the hydrogen sulfite detoxification pathway may increase the risk of UC, a significant risk factor for colon cancer[95,96]. It is interesting to note that taurine conjugation and sulfite production are increased in meat consumers, thus providing another link to colon cancer[2]. In addition, sulfite promotes DCA generation by bacteria through stimulation of 7α-dehydroxylation.
EFFECT OF BA ON ION TRANSPORT
It is well known that BAs elicit fluid secretion and also increased permeability in the gastrointestinal tract[97-101]. BAs also affect intestinal motility, although this field of study has received relatively little attention[1,102]. The effect of BAs on permeability is primarily due to their detergent action on tight junctions, which is reversible. However, at high BA concentrations epithelial lesions may occur. This effect is to a large extent indirect, induced by intramural reflexes containing nicotinic receptors, but probably it does not involve histamine or nitric oxide pathways[97,103,104]. However, this question is controversial, because in some cases histamine has been suggested to be involved[98].
The secretory effect of BAs has been studied in the small and large intestine, and in both cases the mechanism of secretion appears to be largely indirect[99]. In the small intestine BAs elicit serotonin release by enterochromaffin cells in the mucosa by a Ca2+-dependent mechanism, initiating a neural reflex that stimulates ion secretion, as well as inhibited absorption[105]. In ileal perfusion experiments permeability and fluid transport were studied in parallel, finding that the effect is dependent on nicotinic receptors in both cases[97,103]. On the other hand, in the colon BA-induced secretion has been claimed to be prostaglandin dependent[106]. More specifically, prostaglandins may account for the early response to BA stimulation observed in vitro[98]. Mast cells have also been involved in this process[98]. Indeed, BAs have been shown to induce prostaglandin[107] and histamine secretion[108] in vitro. Some of these actions may be secondary to direct mucosal injury[100,109]. Considering the variety of mediators proposed to participate in these events, it is not surprising that multiple intracellular signaling pathways have also been involved.
As with other biological activities of BAs, not all molecular species are equivalent. Dihydroxy BAs, and in particular DCA and CDCA, exhibit prosecretory/antiabsorptive and mucosal damaging effects in the colon, particularly in their unconjugated forms[100,101]. Conversely, other important BAs such as CA and UDCA are generally considered to have no significant bioactivity at this regard. In the small intestine, these differential species-dependent effects are not so well characterized, but they certainly differ from those in the colon. For instance, CDCA is a relatively poor secretagogue in the ileum[110-113]. It should be noted that ileal BA absorption is electrogenic, even in the absence of chloride/bicarbonate secretion, due to the 2:1 stoichiometry of ASBT-mediated Na+:BA symport[111,114,115].
However, BAs also exert direct actions on intestinal epithelial cells. Using the prototypic T84 cell line TDCA was shown to elicit chloride secretion by a Ca2+ dependent mechanism[116]. This pertains to the actions of BAs in the large intestine, since T84 cells have a colonic epithelium phenotype. More recently, BA were described to induce ion secretion via transactivation of the cystic fibrosis transmembrane conductance regulator (CFTR)[113]. CFTR is the main chloride/bicarbonate channel in intestinal epithelial cells and is pivotal to ion secretion in the gastrointestinal tract, among other tissues. Transactivation requires apical colocalization of both CFTR and ASBT, which does occur in the distal ileum but also in cholangiocytes. Moreover, CFTR also plays a role in bile flow, as suggested the presence of plugging and dilatation of bile ducts in cystic fibrosis. Interestingly, BA ileal uptake is compromised in this condition, leading to BA waste and diarrhea[113,114,117], suggesting that CFTR has a reciprocal influence on ASBT. Moreover, this provides an additional meaningful link to inhibited BA absorption in intestinal inflammation, where CFTR has been shown to be downregulated, as is ASBT itself[118,119]. Although the relationship between the expression of CFTR and that of ASBT has been previously demonstrated also for other transporters[120], the underlying mechanism has not been elucidated yet.
One interesting question arises as to what is the physiological role of BA-induced ileal and colonic secretion. The simplest explanation is that ileal secretion, which is evoked in normal conditions, may be useful to aid in intestinal propulsion or to prevent the formation of micelles and the consequent epithelial damage during the absorptive process[113]. In contrast, colonic secretion occurs only in pathologic conditions and may be part of a nonspecific mechanism aimed to eliminate invading microorganisms. In addition, BA ileal absorption is compromised in conditions such as irritable bowel syndrome, Crohn’s disease, cystic fibrosis and surgical resection (short bowel syndrome)[117], producing diarrhea because of the presence of high (millimolar) concentrations in the colonic lumen. BAs (CDCA, UDCA) can also induce diarrhea in their own right when given to gallstone patients[121,122].
BA AND INTESTINAL INFLAMMATION
Certain BAs have been shown to exert intestinal antiinflammatory actions in vivo. Thus UDCA reduces intestinal permeability and oxidative stress in the indomethacin model of ileitis in the rat[123]. Similarly, UDCA counteracts ibuprofen intestinal ulceration in rats[124]. These effects may be related to the actions of BAs on intestinal epithelial cells. Thus DCA induces IL-8 and activates NF-κB in HT29 cells, actions that are opposed by taurine-conjugated UDCA[125,126]. The mechanism for IL-8 induction is probably via the classical NF-κB pathway for DCA and via RelA phosphorylation in the case of TDCA[126]. The stimulatory effect of DCA is reproduced in other[127,128] but not all cell lines[129]. Thus UDCA might exert antiinflammatory actions in the intestine by inhibiting epithelial stimulation. Conversely, DCA is predicted to aggravate inflammation, but this has not been tested. On the other hand, BAs have also been described to enhance epithelial wound healing, an action dependent on NF-κB activation and the release of transforming growth factor β[130]. This effect is shared by TDCA, DCA and TCA. Unfortunately UDCA was not investigated[130]. It cannot be ruled out that this effect may also form part of the mechanism of action of this beneficial compound.
INTESTINAL APPLICATIONS OF BA
The main clinical application of BAs is in the management of gallstones and cholestasis (primary biliary cirrhosis, cystic fibrosis liver disease, drug induced cholestasis)[88,131,132]. UDCA and to a lesser extent CDCA have been used. Cholylsarcosine is an artificial derivative that has been proposed as an alternative. However, BAs have no current application for intestinal conditions. UDCA has been studied in the clinical setting for cancer chemoprevention as discussed above. Specific artificial BA derivatives have also been studied for chemopreventive application[133]. Interestingly, it may be feasible to increase UDCA intestinal exposure by the use of a specific type of probiotic (living bacteria) that epimerizes CDCA to UDCA within the intestinal lumen[134]. BAs have been used in some cases as a replacement to reduce steatorrhea due to short bowel syndrome or secondary to metabolic genetic diseases, frequently at the price of enhanced diarrhea[135,136]. Conversely, diarrhea without steatorrhea benefits from treatment with ion exchange resins such as cholestiramine that bind and act as sequestrant of BAs during their intestinal transit[137].
Moreover, BAs are being considered as galenic agents to improve intestinal absorption of drugs compounds such as nucleotides, heparin or insulin[138-140].
CONCLUSION
Owing to their tensioactive properties BAs play an important role in the intestine, facilitating fat digestion and the absorption of lipids and liposoluble vitamins. Efficient intestinal uptake, mainly at the ileum, permits to recover most of the secreted BA molecules, which are sent back to the liver with the portal blood. The existence of the enterohepatic circulation maintains appropriate levels of BAs ready to be used after meals and prevents exposure of other tissues to high levels of these dangerous detergents. In this respect, due to the potential toxicity of BAs and, at the same time, their biological relevance the homeostasis of BAs is tightly regulated, in part by specific plasma membrane receptors and nuclear receptors. The function, transport and regulatory mechanisms regarding BAs and the intestine have been elucidated in great detail, although some questions remain unanswered, such as the exact physiological role of IBABP. Some BA species have peculiar biological or pharmacological effects, which have been characterized to a great extent. Nevertheless, their role in colon cancer and intestinal inflammation requires further study, which is especially interesting considering the potential therapeutical applications.