Published online Sep 28, 2008. doi: 10.3748/wjg.14.5584
Revised: August 14, 2008
Accepted: August 21, 2008
Published online: September 28, 2008
AIM: To evaluate fecal calprotectin (FC) as a surrogate marker of treatment outcome of relapse of inflammatory bowel disease (IBD) and, to compare FC with fecal myeloperoxidase (MPO) and fecal eosinophil protein X (EPX).
METHODS: Thirty eight patients with IBD, comprising of 27 with ulcerative colitis (UC) and 11 with Crohn’s disease (CD) were investigated before treatment (inclusion), and after 4 and 8 wk of treatment. Treatment outcomes were evaluated by clinical features of disease activity and endoscopy in UC patients, and disease activity in CD patients. In addition, fecal samples were analyzed for FC by enzyme-linked immunosorbent assay (ELISA), and for MPO and EPX with radioimmunoassay (RIA).
RESULTS: At inclusion 37 of 38 (97%) patients had elevated FC levels (> 94.7 μg/g). At the end of the study, 31 of 38 (82%) patients fulfilled predefined criteria of a complete response [UC 21/27 (78%); CD 10/11 (91%)]. Overall, a normalized FC level at the end of the study predicted a complete response in 100% patients, whereas elevated FC level predicted incomplete response in 30%. Normalized MPO or EPX levels predicted a complete response in 100% and 90% of the patients, respectively. However, elevated MPO or EPX levels predicted incomplete response in 23% and 22%, respectively.
CONCLUSION: A normalized FC level has the potential to be used as a surrogate marker for successful treatment outcome in IBD patients. However, patients with persistent elevation of FC levels need further evaluation. FC and MPO provide superior discrimination than EPX in IBD treatment outcome.
- Citation: Wagner M, Peterson CG, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol 2008; 14(36): 5584-5589
- URL: https://www.wjgnet.com/1007-9327/full/v14/i36/5584.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5584
| UC | CD | |
| Number of subjects | 27 | 11 |
| Gender (female/male) | 15/12 | 3/8 |
| Age (yr, mean) | 42.5 (21-66) | 34.6 (21-70) |
| Disease duration (yr, mean) | 9.3 (0-38) | 5.6 (0-18) |
| Extent of disease: | ||
| Colon | 91 | 8 |
| Left side colitis | 14 | - |
| Proctitis | 4 | - |
| Ileocolitis | - | 1 |
| Prior surgery | 1 | 1 |
| Treatment at inclusion: | ||
| No treatment | 7 | 4 |
| 5-ASA (topical/systemic) | 19 | 4 |
| Azathioprine | 1 | 1 |
| Prednisone (topical/systemic) | 5 | - |
| Metronidazole | - | 1 |
| Patients | Fecal marker | Inclusion | 4 wk | 8 wk |
| All | FC and MPO | R = 0.884, P < 0.01 | R = 0.941, P < 0.01 | R = 0.924, P < 0.01 |
| All | FC and EPX | R = 0.592, P < 0.01 | R = 0.724, P < 0.01 | R = 0.642, P < 0.01 |
| UC | FC and MPO | R = 0.868, P < 0.01 | R = 0.927, P < 0.01 | R = 0.940, P < 0.01 |
| UC | FC and EPX | R = 0.556, P < 0.01 | R = 0.762, P < 0.01 | R = 0.602, P < 0.01 |
| CD | FC and MPO | R = 0.936, P < 0.01 | R = 0.818, P < 0.01 | R = 0.903, P < 0.01 |
| CD | FC and EPX | R = 0.654, P < 0.05 | R = 0.636, P < 0.05 | R = 0.854, P < 0.01 |
| Fecal marker | Patients | PPV (95% CI) | NPV (95% CI) |
| FC | All n = 371 | 30 (13-53) | 100 (77-100) |
| FC | UC n = 27 | 38 (13-65) | 100 (71-100) |
| FC | CD n = 101 | 14 (2-58) | 100 (30-100) |
| MPO | All n = 371 | 23 (10-42) | 100 (59-100) |
| MPO | UC n = 27 | 27 (10-50) | 100 (48-100) |
| MPO | CD n = 101 | 12 (2-53) | 100 (19-100) |
| EPX | All n = 371 | 22 (8-42) | 90 (56-98) |
| EPX | UC n = 27 | 25 (8-49) | 85 (42-98) |
| EPX | CD n = 101 | 14 (3-58) | 100 (30-100) |
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic, idiopathic inflammatory condition of the gut with a typically relapsing and remitting course. Exacerbations are characterized by symptoms of diarrhea, urgency of defecation and occasionally rectal bleeding and abdominal pain. The aim of treatment is to induce and maintain disease remission[1]. Currently, the most reliable method to assess intestinal inflammation is endoscopy with mucosal biopsy. These techniques are costly, invasive, time consuming and unpopular with patients. Furthermore, the site of inflammation is not always reached by endoscopy as in the case of small bowel disease in CD. Simple, inexpensive and objective tools for the assessment of mucosal inflammation are therefore desirable. Previous studies have indicated that fecal markers may be used in the differentiation of IBD from functional gastrointestinal disorders. However, the usefulness of these markers in monitoring therapy of patients with disease relapse in IBD needs further evaluation[2-4].
A prominent feature of mucosal histology in patients with active IBD is infiltration by neutrophil granulocytes[5]. Calprotectin is a major protein of neutrophils and macrophages, accounting for about 60% of the cytosol of these cells[6]. Several workers have shown that elevated fecal calprotectin (FC) levels correlate with intestinal inflammation, both in adults and children[7-9], and has the ability to predict relapse in IBD[10,11]. Moreover, FC levels remain stable in stools, and stool samples are easy to collect[12-17].
Other fecal proteins of interest in monitoring IBD are myeloperoxidase (MPO) and eosinophil protein X (EPX). MPO is mainly derived from neutrophil granulocytes and has been observed both in the intestinal mucosa[18-20], and in gut lavage, and has the potential of monitoring treatment outcome[21]. EPX is released by activated eosinophil granulocytes, which are abundant in the mucosa in active IBD[22-25]. We have previously reported that fecal levels of EPX and MPO have the potential of monitoring therapy in UC[26].
The primary aim of the present study was to assess FC as a surrogate marker of treatment outcome of relapse in patients with IBD compared to the standard criteria of response. The second aim was to compare FC with fecal MPO and EPX, with respect to their applicability in monitoring treatment outcome.
Forty adult patients (19 females and 21 males, mean age 40 years, range 21-70 years), seeking medical advice for symptoms of relapse of previously diagnosed IBD were consecutively recruited at the department of Gastroenterology, University Hospital, Uppsala, Sweden, between October 2002 and April 2003. Two patients were excluded: one patient had salmonella enteritis, while the other patient dropout after 8 wk of treatment. Before entry into the study, the patients had to fulfil standard diagnostic criteria of UC (n = 27) and CD (n = 11)[27]. Details of the clinical date of the study population are provided in Table 1.
At inclusion, all patients had mild to moderate disease activity, and the endoscopic examination was consistent with active disease. The exclusion criteria were: pregnant or lactating women, enteritis due to infections, and intestinal biopsies performed within 3 d before inclusion. The project was approved by the Ethical Committee of the Medical Faculty, Uppsala University, and all patients gave their written informed consent before participating in the study.
The patients were examined according to the study protocol at inclusion and after 4 and 8 wk of treatment (Table 2).
In patients with UC, a semi-quantitative four-grade (normal, mild, moderate and severe) scale was used for clinical and endoscopic score[28]. In patients with CD, the Harvey-Bradshaw’s clinical activity index (HBI) was used[29]. Histopathology was graded as active or inactive inflammation, based on the number of neutrophil granulocytes in the mucosa as judged by an experienced pathologist, according to the criteria of Truelove and Witts[30]. At inclusion, 7 of 27 (26%) patients with UC were on no treatment, 14 (52%) were receiving systemic 5-aminosalicylic acid (5-ASA), and 6 (22%) were under treatment with different combinations of systemic 5-ASA, prednisone, azathioprine and topical prednisone. In CD patients, at inclusion 4 of 11 (36%) patient were on no treatment, 6 (54%) were receiving systemic 5-ASA, and one patient was under treatment with a combination of 5-ASA, azathioprine and Metronidazole.
Treatment of a relapse was individualized, according to standard recommendations for the management of IBD. Topical and/or systemic 5-ASA was administered to 26 of 27 (96%) patients with UC, and to 9 of 11 (82%) patients with CD. Topical and/or systemic prednisone was given to 22 of 27 (81%) UC patients, and 7 of 11 (81%) CD patients. Azathioprine was used in 3 of 27 (11%) UC patients and in 3 of 11 (27%) CD patients, while methotrexate was prescribed to one patient with CD.
In UC, a complete response to treatment was defined as return of the clinical and endoscopic scores to normal. A partial response was defined as reduction in both the clinical and endoscopic scores, but their failure to return to normal. A non-response was defined as a decrease in only clinical or endoscopical score or an unchanged or increased clinical and/or endoscopic score. In CD, a complete response was defined as decrease in HBI score to ≤ 5 points; partial response was a decrease in the clinical score, but not < 6 points; and a non-response was defined as an unchanged or increased HBI score.
Stool samples were collected in screw-capped plastic containers at inclusion (visit 1), and after four (visit 2) and 8 wk of treatment (end of study, visit 3). Stool samples were kept at 4°C for up to 2 d before freezing at -70°C. Stool extracts for EPX and MPO measurement were prepared as described previously[31]. Calprotectin in fecal extracts was analyzed, using the calprotectin enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (Calprest; Eurospital SpA, Trieste, Italy). Before testing, the supernatants were thawed, diluted 1:50 with assay buffer and then analyzed with Calprest. Calprotectin was expressed as microgram per gram of feces. According to the manufacturer, a calprotectin level > 50 μg/g is pathological. FC was determined in samples from 44 apparently healthy adults, and the normal range was 9.2-94.5 μg/g (5-95th percentile). EPX and MPO were determined using radioimmunoassay (RIA) (Pharmacia Diagnostics, Uppsala, Sweden), and the intra- and inter-assay variations were less than 9% for both assays. Marker levels in faeces were adjusted for fecal water content, as described previously[31], and were expressed as µg/g semidry faeces. The concentration of fecal markers in healthy adults for fecal MPO (1.3-8.8 μg/g, 5-95th percentile) and fecal EPX (0.2-1.7 μg/g, 5-95th percentile) (median age 44 years, range 18-73, n = 44) have been reported recently[31].
Non-parametric tests of Kruskal-Wallis ANOVA and the Mann-Whitney U-test were used for unpaired comparisons. For paired analyses we used Friedman ANOVA and Wilcoxon matched pairs test. Spearman rank order correlations were used to express relationship between variables. P < 0.05 was considered as significant. All calculations were performed on a personal computer by means of the statistical software Statistica (Statsoft Inc, Tulsa, Oklahoma USA). We used the 95th percentile concentration level of respective fecal marker as the cut-off level for normal and elevated values i.e. concentration was set at 94.5 μg/g for FC, at 8.8 μg/g for MPO, and at 1.7 μg/g for EPX.
At inclusion, 37 of 38 (97%) patients manifested elevated FC levels. There was no difference in the FC levels between UC and CD at any time point during the study. Overall, a significant decline in FC levels was seen during the study (P < 0.01) in patients with UC (P < 0.01), but not in CD (P = 0.367) (Table 3).
Analysis based on the extent of the disease showed that patients with proctitis had lower levels of FC (P < 0.01) compared with more extensive disease. This difference was noted in patients with UC (P < 0.05), but was not analyzed in CD since only one patient had proctitis.
In patients with UC, there was no correlation between the clinical score and FC levels at inclusion, and at the end of the study (8 wk of treatment). However, correlation was noted after 4 wk of treatment (R = 0.0424, P < 0.01).
In CD patients, there was no correlation between FC and the HBI score at inclusion, and after 4 wk of treatment, but correlation was present at the end of the study (8 wk of treatment) (R = 0.7804, P < 0.01).
In patients with UC, the endoscopic score was calculated and correlated with FC levels. No correlation was seen at inclusion, but after 4 and 8 wk of treatment a correlation was noted (R = 0.5045, P < 0.01) and (R = 0.6659, P < 0.01), respectively.
At inclusion, all patients with UC (27/27) showed histological signs of active disease, whereas 12 of 26 (one missing value) and 13 of 27 showed inactive histology after 4 and 8 wk of treatment, respectively. Patients with UC with histologic evidence of active inflammation, demonstrated higher levels of FC compared to UC patients with inactive inflammation after 4 and 8 wk of treatment (P < 0.05). In CD patients, no difference was found during the study period.
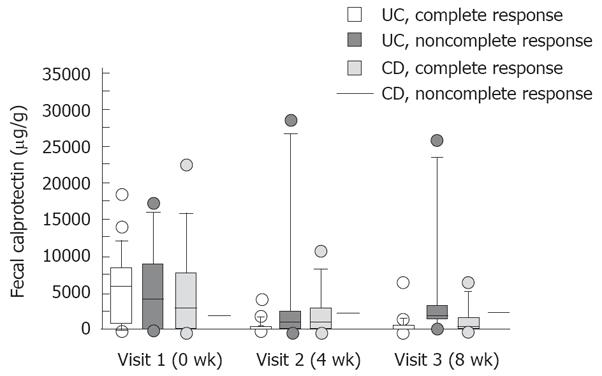
In UC patients, a complete response was observed in 14 of 27 (52%) and 21 of 27 (78%) patients after 4 and 8 wk of treatment, respectively. Two patients were classified as partial responders and four patients as non responders at the end of the study. In complete responders, there was a significant decline in FC levels (P < 0.01), which was not observed in partial or non responders (Figure 1). The decline in FC levels was already significant after 4 wk of treatment in complete responders (P < 0.01).
In CD patients, a complete response was observed in 9 of 11 (81%) and 10 of 11 (91%) patients after 4 and 8 wk of treatment, respectively. There was a trend in the decline in FC levels in complete responders but the difference was not significant (P = 0.13) (Figure 1). One CD patient, who was a partial responder, manifested increasing levels of FC during the study (2030, 2220, 2460 μg/g, respectively).
There was a good correlation between FC, and both MPO and EPX, at all the study visits in the group as a whole, as well as on sub-group analyses of patients with UC and CD (Table 4).
In order to predict treatment outcome, positive predictive value (PPV) and negative predictive value (NPV) were calculated at the end of the study (after 8 wk of treatment). All patients with a normalized FC value at the end of the study fulfilled the predefined criteria of a complete response, irrespective of the diagnosis of UC or CD. However, an elevated FC value was noted in 10 of 21 patients with UC and 6 of 9 patients with CD who fulfilled criteria for complete response at the end of the study. The same pattern was observed in patients with UC and CD with regard to MPO, however, for EPX this was only seen in CD patients (Table 5).
In the present study, we evaluated FC as a surrogate marker of treatment outcome in IBD. FC levels were monitored before and during treatment of patients with a relapse of IBD. In both UC and CD, patients with normalized FC level after 8 wk of treatment fulfilled predefined criteria of a complete response. However, elevated FC levels at the end of the study did not rule out response to treatment. As described by other workers, we also observed elevated FC levels in all but one patient presenting with disease relapse[8,9].
It has been reported that FC levels in UC patients reflects the degree of inflammation rather than the extent of the disease[8]. By contrast, in the present study, we observed higher FC levels in UC patients at inclusion with extensive disease compared to those with proctitis.
Endoscopic findings have been shown to correlate with FC levels in patients with IBD[32]. In the present study, no such correlation was observed in UC patients at inclusion, however, a correlation was noted after 4 and 8 wk of treatment. It is well known that there are difficulties in endoscopic grading of inflammation due to inter-observer variations[33], and with the use of different scoring systems. In the present study, we used a four-grade score[28], which may be inadequate in distinguishing between mild and moderate inflammation.
In patients with UC, the clinical activity index and FC levels showed a correlation after 4 wk of treatment, but not at inclusion or at the end of the study. In patients with CD, clinical activity and FC showed a correlation only at the end of the study. These weak correlations between clinical indices and FC levels are in accordance with previous reports[9,34,35]. By contrast, a recent study reported significantly higher FC levels in patients with severe disease compared to those with moderate disease[7]. Since our patients manifested only mild to moderate disease, the present study cannot clarify this matter.
The histological findings in present study confirmed previous reports that patients with active UC manifest higher FC levels compared to those with inactive disease[8,32]. However, this finding was not seen in CD patients. These observations should be interpreted with caution, since the number of patients was small. One may speculate that one explanation for these findings may be that biopsies are more prone to sampling error in CD because of the patchy mucosal inflammation in CD compared to UC. Using FC values below ULN (95th percentile of the normal range) as a negative predictor of active disease after 8 wk of treatment, revealed a NPV of 100%. This is in line with previous reports showing that normalisation of FC predicts mucosal healing in patients with IBD[4].
However, using an elevated level of FC as a positive predictor to detect ongoing active disease or treatment failure after 8 wk of treatment, showed a PPV of 38% in UC and only of 14% in CD. In children with active IBD treated with prednisone, FC levels declined in line with clinical improvement but seldom fell within the normal range[36]. A persistently high level of FC suggests ongoing inflammation in clinically silent disease and may predict relapse of IBD[11,37]. It is possible that our patients who responded to therapy but continued to have elevated FC, may have normalized the FC levels if the study had been prolonged. Another explanation is that these patients may relapse again soon after termination of the study. After the completion of the present study, we followed our patients retrospectively but did not find any relapsers over the following 3 mo (data not shown). At present, there is insufficient data to conclude that patients without clinical or endoscopic signs of active IBD but with elevated FC levels, may benefit from more aggressive anti-inflammatory treatment.
In the present study, fecal MPO and EPX were also evaluated in order to monitor treatment outcome compared with FC levels. A recent study showed that MPO and EPX may be used to detect active UC[26]. In the present study, we observed a close correlation between FC, MPO and EPX, and treatment outcome; FC and MPO provided better assessment of treatment outcome compared to EPX in patients with UC. Interestingly, normalized levels of EPX after 8 wk of treatment indicated a complete response, especially in patients with CD.
In conclusion, normalized FC level may be used as a surrogate marker for treatment response in patients with IBD. However, the significance of persistent elevation of FC needs further evaluation.
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic, idiopathic inflammatory conditions of the gut with a typically relapsing and remitting course. Exacerbations are characterized by symptoms of diarrhea, urgency of defecation, and occasionally rectal bleeding and abdominal pain. At present, the most reliable method of assessing intestinal inflammation is endoscopy with biopsy. These techniques are costly, invasive, time consuming and unpopular with the patients. Furthermore, the site of inflammation is not always reached by endoscopy as in patients with CD of the small bowel. Simple, inexpensive and objective tools for the assessment of mucosal inflammation are therefore desirable. Previous studies have indicated that fecal markers may be used in the differentiation of inflammatory bowel disease (IBD) from functional gastrointestinal disorders. However, the usefulness of these markers in monitoring therapy of patients with relapse of IBD needs further evaluation.
The present study suggests that a normalized fecal calprotectin (FC) level may be used as a surrogate marker for successful treatment outcome in IBD patients. However, patients with persistently elevated FC levels need further evaluation.
The present study confirmed previous observations that patients with a relapse of IBD had elevated fecal markers, and normalized FC levels may be used as a surrogate marker for treatment response in IBD. We evaluated patients diagnosed previously with UC or CD before starting treatment, and after 4 and 8 wk of treatment. Treatment outcome, based on clinical activity and endoscopic findings in UC patients, and clinical activity in CD patients, were evaluated together with fecal samples analyzed for FC, and compared with fecal myeloperoxidase (MPO) and eosinophil protein X (EPX). It was observed that FC and MPO provided better assessment of treatment outcome compared to EPX in patients with UC. Interestingly, normalized levels of EPX after 8 wk of treatment indicated a complete response, especially in patients with CD.
These findings suggest that fecal markers can be used as surrogate markers for successful treatment outcome in IBD patients. Fecal markers are simple, inexpensive and objective tools for the assessment of mucosal inflammation.
This study provides good data on material and methods. The results are based on sufficient experimental evidence. The discussion is well organized, and the conclusions are reliable and valuable.
Peer reviewer: Riina Salupere, MD, PhD, Division of Endocrinology and Gastroenterology, University of Tartu, L. Puusepa Street 6, Tartu 51014, Estonia
S- Editor Li DL L- Editor Anand BS E- Editor Zhang WB
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. |
| 2. | Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:247-255. |
| 3. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. |
| 4. | Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017-1020. |
| 5. | Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121-1128. |
| 6. | Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113-123. |
| 7. | Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, Dobos GJ. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085-1091. |
| 8. | Roseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176-180. |
| 9. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506-513. |
| 10. | Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642-647. |
| 11. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. |
| 12. | Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, Scalici C, Montalto G, Di Fede G, Rini G. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861-867. |
| 13. | Dale I, Brandtzaeg P, Fagerhol MK, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985;84:24-34. |
| 14. | Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. |
| 15. | Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50-54. |
| 16. | Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841-845. |
| 17. | Fagerberg UL, Loof L, Lindholm J, Hansson LO, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414-420. |
| 18. | Carlson M, Raab Y, Seveus L, Xu S, Hallgren R, Venge P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut. 2002;50:501-506. |
| 19. | Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C. Interleukin-8 and neutrophil markers in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol. 1992;87:1447-1452. |
| 20. | Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927-934. |
| 21. | Sangfelt P, Carlson M, Thorn M, Loof L, Raab Y. Neutrophil and eosinophil granule proteins as markers of response to local prednisolone treatment in distal ulcerative colitis and proctitis. Am J Gastroenterol. 2001;96:1085-1090. |
| 22. | Bischoff SC, Wedemeyer J, Herrmann A, Meier PN, Trautwein C, Cetin Y, Maschek H, Stolte M, Gebel M, Manns MP. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1-13. |
| 23. | Raab Y, Fredens K, Gerdin B, Hallgren R. Eosinophil activation in ulcerative colitis: studies on mucosal release and localization of eosinophil granule constituents. Dig Dis Sci. 1998;43:1061-1070. |
| 24. | Carlson M, Raab Y, Peterson C, Hallgren R, Venge P. Increased intraluminal release of eosinophil granule proteins EPO, ECP, EPX, and cytokines in ulcerative colitis and proctitis in segmental perfusion. Am J Gastroenterol. 1999;94:1876-1883. |
| 25. | Levy AM, Gleich GJ, Sandborn WJ, Tremaine WJ, Steiner BL, Phillips SF. Increased eosinophil granule proteins in gut lavage fluid from patients with inflammatory bowel disease. Mayo Clin Proc. 1997;72:117-123. |
| 26. | Peterson CG, Sangfelt P, Wagner M, Hansson T, Lettesjo H, Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest. 2007;67:810-820. |
| 27. | Garland CF, Lilienfeld AM, Mendeloff AI, Markowitz JA, Terrell KB, Garland FC. Incidence rates of ulcerative colitis and Crohn's disease in fifteen areas of the United States. Gastroenterology. 1981;81:1115-1124. |
| 28. | Binder V. A comparison between clinical state, macroscopic and microscopic appearances of rectal mucosa, and cytologic picture of mucosal exudate in ulcerative colitis. Scand J Gastroenterol. 1970;5:627-632. |
| 31. | Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755-1762. |
| 32. | D'Inca R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429-437. |
| 33. | Baron JH, Connell AM, Lennard-Jones JE. Variation between Observers in Describing Mucosal Appearances in Proctocolitis. Br Med J. 1964;1:89-92. |
| 34. | Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986;27:92-95. |
| 35. | Korelitz BI. An indicator of Crohn's disease activity. A need unfulfilled. J Clin Gastroenterol. 1986;8:220-222. |
| 36. | Kolho KL, Raivio T, Lindahl H, Savilahti E. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;720-725. |