Published online Sep 21, 2008. doi: 10.3748/wjg.14.5412
Revised: May 25, 2008
Accepted: June 1, 2008
Published online: September 21, 2008
AIM: To investigate the biological impact of hepatitis B virus X- hepatitis C virus core (HBV X-HCV C) fusion gene on hepatoma cells.
METHODS: The recombinant adenoviruses Ad-XC, Ad-X and Ad-C expressing HBV X-HCV C fusion gene, HBV X gene and HCV C gene were constructed, respectively. Hepatoma cells were infected with different recombinant adenoviruses. MTT, colony-forming experiment, FCM, TUNEL assay were performed to observe the biological impact of the HBV X-HCV C fusion gene on liver cells.
RESULTS: MTT showed that the Ad-XC group cells grew faster than the other group cells. Colony-forming experiment showed that the colony-forming rate for the Ad-XC group cells was significantly higher than that for the other group cells. FCM analysis showed that Ad-XC/Ad-X/Ad-C infection enhanced the progression of G1→S phase in the HepG2 cell cycle. The apoptosis index of the Ad-XC, Ad-X, Ad-C group cells was significantly lower than that of the Ad0 and control group cells. Semi-quantitative RT-PCR showed that the expression level of c-myc was the highest in Ad-XC infected cells. Tumor formation was found at the injected site of mice inoculated with Ad-XC-infected LO2 cells, but not in control mice.
CONCLUSION: Ad-XC, Ad-X and Ad-C facilitate the proliferation activity of HepG2 cells and inhibit their apoptosis in vitro. The effect of Ad-XC is significantly stronger than that of Ad-X and Ad-C. Up-regulation of c-myc may be one of the mechanisms underlying the synergism of HBV X and HCV C genes on hepatocarcinogenesis in athymic nude mice.
- Citation: Ma Z, Shen QH, Chen GM, Zhang DZ. Biological impact of hepatitis B virus X-hepatitis C virus core fusion gene on human hepatocytes. World J Gastroenterol 2008; 14(35): 5412-5418
- URL: https://www.wjgnet.com/1007-9327/full/v14/i35/5412.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5412
| Target gene | Primer sequences | Product (bp) |
| HBV X gene | P1: 5’-ATCTGTCGACATGGCTGCTAGGCTGTGCT G-3’ | 465 |
| P2: 5’-CGCGGATATCTTAGGCAGAGGTGAAAAAGT TG-3’ | ||
| HCV C gene | P3: 5’-ACTGGTCGACATGAGCACGAATCCTAAACCT C-3’ | 576 |
| P4: 5’-ACTGGATATCTTAGGCTGAAGCGGGCAC AG-3’ | ||
| HBV X-Linker fragment | P1: 5’-ATCTGTCGACATGGCTGCTAGGCTGTGCT G-3’ | 510 |
| P2’: 5’-GCTGCCGCCACCGCCGCTTCCGCCACCGCCGCTTGCCACCGGCAGAGGTGAAAAAGTTGCA-3’ | ||
| Linker-HCV C fragment | P3’: 5’-GGTGGCGGTGGAAGCGGCGGTGGCGGCGGAAGCGGCGGTGGCGGCAGCATGAGCACGAATCCTAAACCTC -3’ | 621 |
| P4: 5’-ACTGGATATCTTAGGCT GAAGCGGGCAC AG-3’ | ||
| HBV X-HCV C fusion gene | P1: 5’-ATCTGTCGACATGGCTGCTAGGCTGTGCT G-3’ | 1086 |
| P4: 5’-ACTGGATATCTTAGGCTGAAGCGGGCAC AG-3’ | ||
| c-myc | 5’-TTCGGGTAGTGGAAAACCAG-3’ | 203 |
| 5’-CAGCAGCTCGAATTTCTTCC-3’ | ||
| β-actin | 5’-GTGGGGCGCCCCAGGCACCA-3’ | 540 |
| 5’CTTCCTTAATGTCACGCACGATTTC-3’ |
Hepatocellular carcinoma (HCC) is the second most common cancer in China. Many etiological factors are related with HCC development. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) and prolonged dietary exposure to aflatoxin are responsible for about 80% of all HCCs in human beings. Chronic HBV and HCV infection often results in cirrhosis and enhances the probability of developing HCC. The underlying mechanisms leading to malignant transformation of infected cells, however, remain unclear. Based on epidemic data, super-infection with HBV and HCV is associated with the increased frequency in the development of HCC, but the relative mechanism remains elusive. It has been reported that both HBV X gene and HCV core (HCV C) gene play an important role in hepatocarcinogenesis. The fact that HBV X and HCV C genes induce HCC in transgenic mice offers more evidence for the relationship between these genes and HCC.
Imbalance between proliferation and apoptosis may contribute to hepatocarcinogenesis. HBV X and HCV C proteins are multiple-functional proteins, and can deregulate cell cycle check points, transactivate or activate cellular and viral genes, which are involved in transcription regulation, signal transduction pathway, cell cycle regulation, etc. Therefore, they may deregulate cell cycle and apoptosis and may have a common target point. However, if there is synergism of HBV X and HCV C proteins on hepatocarcinogenesis still remains unclear. In the present study, recombinant adenoviruses expressing HBV X-HCV C fusion protein, HBV X protein and HCV C protein were constructed, and their effect on the biological behavior and expression of c-myc in hepatocytes were investigated.
PyrobestTM DNA polymerase, SalI, EcoRV, HindIII and T4 DNA ligase were purchased from TaKaRa Company (Dalian, China). Plasmid pecob6 was constructed by professor Ren Hong in our institute, plasmid pcDNA3.1/HCV-C was constructed by Dr. Wei-xian Chen in our institute. Lipofectamine was purchased from Invitrogen Company (California, USA). PacI and PmeI were from New England Biolabs (Beijing, China). Primers were synthesized by Shanghai Sangon Company (Shanghai, China). AdEasy system was a gift from professor Tong-Chuan He, University of Chicago Medical Center. DH5α was kept in our laboratory. Fetal bovine serum and calf serum were purchased from Hyclone (Utah, USA). RPMI 1640 was provided by GIBCO (New York, USA). 293 cells, LO2 and HepG2 cells were purchased from Shanghai Cytobiology Research institute of Chinese Academy of Science (Shanghai, China). BALB/c nude mice were from Shanghai Experimental Animal Centre of Chinese Academy of Science. Mouse anti-human hepatitis B virus X-protein monoclonal antibody was purchased from CHEMICON (California, USA). Monoclonal antibody to hepatitis C virus core protein was purchased from BIODESIGN (Maine, USA).
The recombinant adenoviruses Ad-XC, Ad-X and Ad-C containing HBV X-HCV C fusion gene, HBV X gene and HCV C gene, were constructed using the AdEasy system[1]. HBV X and HCV C genes were amplified by PCR from pecob6 or pcDNA3.1-HCV C. Using gene SOEing method[2], HBV X and HCV C genes were fused through a linker coding for a sequence rich in glycine. The sequences of gene primers used in this study are listed in Table 1. The fragment was bi-mold-cut with SalI and EcoRV and inserted into two spots of SalI and EcoRV of the pAdTrack-CMV. The recombinant shuttle plasmid was confirmed by PCR, double restriction nuclease digestion and sequencing. Mini preparations were performed using the conventional alkaline lysis method. The linearized shuttle plasmid was co-transformed with adenoviral backbone plasmid pAdEasy-1 to E. coli BJ5183 by electroporation. The cloned candidate was further tested by restriction nuclease digestion with Pac I and PCR. After digested with PacI, the recombinant adenoviral plasmid was transfected into 293 cells for package. Generation of recombinant adenoviruses was monitored by GFP expression. Transfected cells were collected 12-15 d after transfection by scraping cells off flasks and pelleting them with 1 mL PBS. After three cycles of freezing and rapid thawing at 37°C, 10 μL proteinase K was added into 5 μL of viral lysate at 55°C for 1 h and boiled for 5 min and 2 μL of which was used as a model to identify the correct recombinant adenovirus (HBV X, HCV C and HBV X-HCV C fusion gene). The upstream sequence of adenovirus primer is 5'-CTGTGGACCGTGAGGATA-3', the downstream sequence of adenovirus primer of adenovirus primer is 5'-TGTTGGGCATAGATTGTT-3' (Table 1). PCR system contained 2 μL viral DNA, 2 μL 10 μmol/L primer, 5 μL 10 × PCR buffer, 3 μL MgCl2, 4 μL 2 mmol/L dNTP and 1 μL Taq enzyme. Water was added until the final volume of PCR reached 50 μL. Thirty-five cycles of PCR were carried out, each consisting of 94°C for 30 s, 51°C for 40 s, 72°C for 40 s, and a final extension at 72°C for 10 min. Electrophoresis on 1.0% agarose gel was performed. The identified positive recombinant adenoviruses were amplified in 293 cells for further experiment. The virus titer was tested with GFP expression and limited dilution method.
Two hundred and ninety three, NIH3T3 and HepG2 cells were seeded in six-well plates in the appropriate medium at a density of 2 × 105 cells/well, infected with various adenoviruses at a multiplicity of infection (MOI) from 5 to 100, and incubated at 37°C for 48 h. The number of cells expressing GFP was recorded under an inversion difference fluorescence microscope. The percentage of cells with GFP expression was calculated.
The human liver cancer cell line HepG2 was incubated in an atmosphere containing 50 mL/L CO2 at 37°C. When the density reached 90%, the experimental groups were infected with Ad-XC, Ad-X, Ad-C or Ad0, respectively, at different MOI. During the process, the culture fluid was shaken every 30 min, 4 h later. Another 6 mL 10% NCS RPMI 1640 was added. The control group was a non-virus group. After HepG2 cells were incubated for 48 h, total protein was extracted using RIPA. The expression of HBV X-HCV C fusion protein, HBV X and HCV C protein was detected by Western blot.
HepG2 cells (1.5 × 103 cells/well) were plated in 96-well plates (16 wells for each group) and infected with various MOI of Ad0, Ad-C, Ad-X or Ad-XC, respectively. Cell proliferation was determined by MTT assay. After 1-7 d, 20 μL of MTT solution (5 mg/mL) was added to each well. After incubation for 4 h at 37°C, MTT was removed and 200 μL dimethyl sulfoxide (Sigma) was added. The mixture was shaken and the crystals were fully dissolved for about 10 min. The A value of each well was detected at a test wavelength of 490 nm. Cell growth curve was plotted according to the A values.
HepG2 cells of five groups were digested into a single-cell suspension and inoculated into a six-well plate. Each well was inoculated with 1.0 × 103 cells. The cells were incubated for 12 d and fixed with methanol, stained with Giemsa stain fluid. Then the number of colonies with more than 50 cells was recorded. The experiment was repeated five times.
When the density of HepG2 cells reached 90%, the experimental groups were infected with Ad-XC, Ad-X, Ad-C and Ad0, respectively. Forty-eight hours after infection, the cells were collected (using trypsin digestion) and centrifuged at 1000 r/min for 5 min. The upper clear fluid was discarded, PBS was added to adjust the cell density to 106/mL. One hundred microliters of cell suspension was put into a tube, into which 200 μL DNA-PREPTMLPR was added and mixed. Two microliters of DNA-PREPTM stain reagent (PI stain) was added and mixed after 30 s. After 30 min, the cell cycle was detected by flow cytometry (FCM).
Cell apoptosis was estimated by TUNEL staining. HepG2 cells were planted into 24-well plates at a density of 1 × 105 cells/well and infected with Ad-XC, Ad-X, Ad-C and Ad0 respectively. At the same time, 1 mL TNF-α (100 ng/mL) was added into each well. After incubation at 37°C for 48 h, the cells were fixed with 4% paraformaldehyde and chilled in ice bath for 4 min with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate). Then, 50 μL of TUNEL mixture was added, incubated in a humidified chamber at 37°C for 90 min. The TUNEL mixture was removed, 50 μL POD was added and incubated for another 40 min. The cells were rinsed with PBS, stained with DAB, and detected by optic microscopy.
Expression of c-myc mRNA in each group was assayed by semi-quantitative RT-PCR. β-actin was used as an internal control. Total RNA was extracted with TRIZOL reagent. RT-PCR was performed using an access RT-PCR system (Promega). The reaction volume was 50 μL containing 10 μL AMV/Tf1 buffer, 2 μL MgSO4, 1 μL dNTP, 1 μL target gene sense and anti-sense primers, 1 μL β-actin primer pair, 1 μL AMV reverse transcriptase, 1 μL Tf1 DNA polymerase, 2 μg RNA and nuclease-free water. The sequences of gene primers are listed in Table 1. Thirty cycles of amplification were performed, each consisting of denaturation at 94°C for 45 s, annealing at 58°C for 30 s, extension at 37°C for 1 min, an initial denaturation at 45°C 45 min and at 94°C for 2 min, and a final extension at 72°C for 10 min. About 5 μL PCR products were separated by electrophoresis on 10 g/L agarose gel and detected by ultraviolet radiography. The densities of bands were analyzed using a Bio imaging system, the ratio of c-myc density to β-actin density was represented as the relative expression level of mRNA. The semi-quantitative detection was analyzed five times.
LO2 cells were infected with Ad0 or Ad-XC. The infected cells were collected and resuspended in 200 μL PBS after 48 h. Three BALB/c nude mice were subcutaneously inoculated with the infected LO2 cells randomly (Ad0, n = 1; Ad-XC, n = 2). Tumors were observed every 2 d for 6 wk.
All data were expressed as mean ± SE. The significance for the difference between groups was assessed with SPSS 12.0 by one-way ANOVA. P < 0.05 was considered statistically significant.
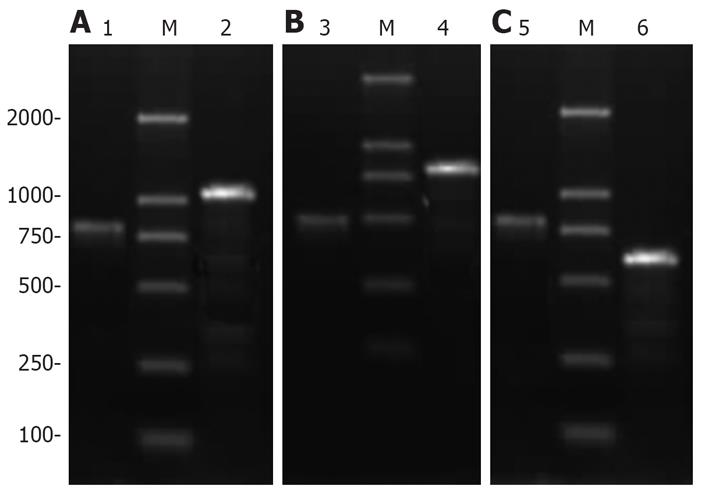
The PCR product depending on the viral DNA model was evaluated by 1.0% agarose electrophoresis. The recombinant adenoviruses that could amplify 465 bp HBV X cDNA fragment/576 bp HCV C cDNA fragment/HBV X-HCV C fusion gene fragment and a 759 bp virus gene frame fragment at the same time were obtained (Figure 1). Recombinant adenoviruses containing HBV X cDNA fragment, HCV C cDNA fragment and HBV X-HCV C fusion gene fragment, respectively, were produced.
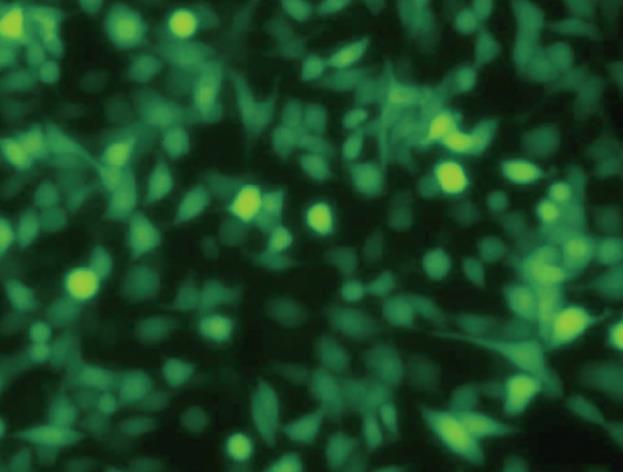
The titer of amplified recombinant adenoviruses Ad-XC, Ad-X, Ad-C and Ad0 was 1.9 × 109 pfu/mL, 2.0 × 109 pfu/mL, 2.2 × 109 pfu/mL, 1.7 × 109 pfu/mL, respectively. When the MOI was 20 or greater, the infection rate of HepG2 cells reached 100% (Figure 2).
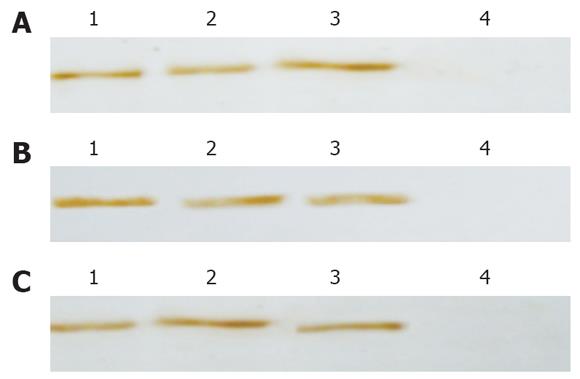
Forty-eight hours after infection, Western blot revealed the expression of HBV X-HCV C fusion protein, HBV X and HCV C proteins in HepG2 cells infected with recombinant adenovirus (Figure 3).
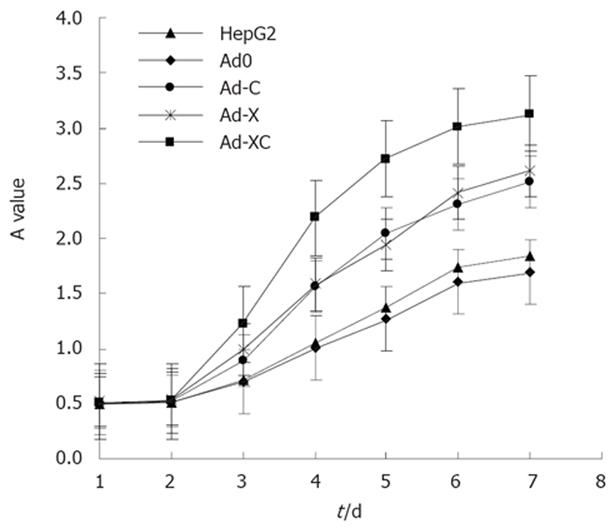
The expression of HBV X-HCV C fusion gene, HBV X and HCV C genes improved cell proliferation significantly compared with that of control HepG2 cells. The A values were higher in HepG2 cells infected with Ad-XC, Ad-X and Ad-C than in control HepG2 cells (Figure 4). These results indicate that HBV X-HCV C fusion gene, HBV X and HCV C genes, especially Ad-XC, stimulated the metabolic activity and the viability of HepG2 cells.
The colony-forming rate of Ad-XC infected HepG2 cells was 82.2% ± 6.1%, significantly higher than that of the Ad-X, Ad-C, Ad0 infected HepG2 cells and control cells (53% ± 4.1%, 49% ± 7.1%, 27.6% ± 5.1%, 30.2% ± 4.4%, respectively, P < 0.0001, n = 5).
Cell cycles from FCM are listed in Table 2. Compared with Ad0 and non-virus group cells, the number of cells in G0/G1 phase was decreased, but increased in Ad-XC, Ad-X, Ad-C groups at S phase, indicating that proliferation of the cells was active. A significant difference was observed in cell proliferation between the Ad-XC group and other groups (P < 0.0001, n = 3).
TUNEL showed that the apoptosis rate of HepG2, Ad0, Ad-C, Ad-X and Ad-XC cells was 20.7% ± 0.6%, 21.8% ± 0.9%, 12.6% ± 0.8%, 11.7% ± 0.9% and 5.1% ± 0.8%, respectively. The apoptosis rate of the experimental group decreased obviously in comparison to the control group. The apoptosis rate of Ad-XC was the lowest. The apoptosis rate of these five groups of cells differed sharply when compared to each other (P < 0.0001, n = 3).
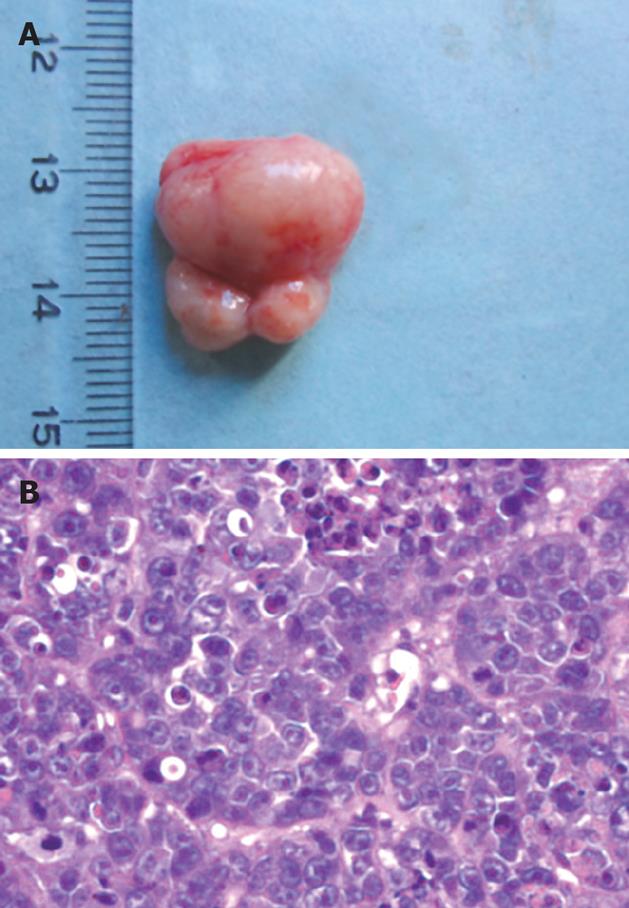
Tumor formation was observed at the injection site of mice inoculated with Ad-XC infected LO2 cells (Figure 5), but not in control mice.
The mRNA level of c-myc in Ad-XC cells was the highest (Figure 6), indicating that transient expression of HBV X-HCV C fusion gene obviously induced expression of c-myc in HepG2 cells.
HCC is one of the most common malignant tumors in the world. Chronic hepatitis B and C are responsible for the great majority of cases of HCC worldwide. Both HBV and HCV are parenterally transmitted and superinfection is not uncommon in intravenous drug users and in countries with a high prevalence of HBV[3]. The risk of developing HCC in subjects with both HBV and HCV infections has been investigated in two meta-analyses[4,5], showing that there is a synergistic hepatocarcinogenic interaction between HBV and HCV infections and that the increased risk is super-additive but not multiplicative.
It was reported that transgenic mice with hepatitis B and C have the oncogenic potential of HBV X and HCV C genes in the liver[6,7]. It was also reported that HBV X and HCV C proteins have an oncogenic potential[8-14], but the involvement of their synergisms in hepatocarcinogenesis remains unclear. HBV X and HCV C proteins additively repress the universal cyclin-dependent kinase inhibitor p21 gene at the transcription level and additively stimulate cell growth, suggesting that additive repression of p21 is important to understand the cooperative development of HCC due to these two proteins[15]. When HBV X and HCV C proteins transform mouse fibroblast NIH3T3 cells in cooperation, they additively stimulate cell growth, especially in the absence of serum growth factors. Cells expressing these two viral proteins exhibit a higher tumorigenicity, as demonstrated in athymic nude mice[16]. HBV X protein increases liver pathogenesis in HCV transgenic mice by a mechanism involving an imbalance between hepatocyte death and regeneration[17]. In the present study, Ad-XC, Ad-X and Ad-C could facilitate the proliferation activity of HepG2 cells and inhibit their apoptosis in vitro. The effect of Ad-XC was significantly stronger than that of Ad-X and Ad-C, suggesting a more than additive but less than multiplicative effect of HBV X and HCV C genes on hepatocarcinogenesis as demonstrated in athymic nude mice[16].
The increased expression of oncogene is thought to be a major cause for tumor formation/progression. C-myc, an oncogene located on 8q24, may be important in hepatocarcinogenesis. The expression level of c-myc in the cells transiently transfected with the HBV X gene was much higher than that in the control cells[18]. c-myc protein expression above its basal level significantly increased c-myc stability, as revealed by its prolonged intracellular half-life in HepG2 expressing HCV core protein, suggesting that HCV core protein may promote cell cycle progression in HepG2 cells by increasing the stability of c-myc oncoprotein[19]. The present study aimed to evaluate the expression level of c-myc in cells infected with different recombinant adenoviruses by RT-PCR. The highest expression level of c-myc was observed in Ad-XC infected cells, suggesting that up-regulation of c-myc expression may be one of the mechanisms underlying the synergism of HBV X and HCV C genes in hepatocarcinogenesis.
In conclusion, HBV X and HCV C genes have a synergism in hepatocarcinogenesis. The reasons for the interaction are uncertain, although the increased c-myc expression in the presence of both genes with tumor promoting effects, including the enhanced up-regulation of c-myc expression, may play a role in hepatocarcinogenesis. Interaction between hepatocarcinogenic effects of the two genes remains to be investigated.
Chronic hepatic B virus (HBV) and hepatic C virus (HCV) infection often results in cirrhosis and enhances the risk of developing HCC. The underlying mechanism leading to malignant transformation of infected cells, however, remains unclear. Based on epidemic data, Super-infection with HBV and HCV is associated with an increased frequency in the development of HCC, but the relative mechanism remains to be elucidated. It was reported that both HBV X and HCV core (HCV C) genes play an important role in hepatocarcinogenesis.
The fact that HBV X and HCV C genes induce HCC in transgenic mice offers more evidence for the relationship between these genes and HCC. However, whether there is a synergism of HBV X and HCV C proteins on hepatocarcinogenesis is still unclear.
In the present study, recombinant adenoviruses expressing HBV X-HCV C fusion protein were constructed, and their effects on biological behavior and c-myc expression level in hepatocytes were investigated.
HBV X and HCV C genes may have a synergism in hepatocarcinogenesis. The reasons for the interaction are uncertain, although increased c-myc expression in the presence of both genes with tumor promoting effects, including the enhanced up-regulation of c-myc expression, may play a role in hepatocarcinogenesis. Interaction between hepatocarcinogenic effects of the two genes remains to be investigated.
This is an elegant, well designed study investigating the combined effect of adenoviruses expressing HBV-X and HCV-C genes on the proliferation and apoptosis of HepG2 cells. The major finding of the study was that the combined effect of the two genes on cell proliferation and apoptosis was superior over that of HBV-X or HCV-C gene alone. The authors also stressed that the underlying mechanism may be, at least, partly explained by the increased c-myc expression.
Peer reviewer: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary
S- Editor Zhong XY L- Editor Wang XL E- Editor Ma WH
| 1. | He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509-2514. |
| 2. | Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61-68. |
| 3. | Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, Seigneurin JM, Buffet C, Dhumeaux D. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33. |
| 4. | Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347-354. |
| 5. | Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607-612. |
| 6. | Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317-320. |
| 7. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. |
| 8. | Zhang JL, Zhao WG, Wu KL, Wang K, Zhang X, Gu CF, Li Y, Zhu Y, Wu JG. Human hepatitis B virus X protein promotes cell proliferation and inhibits cell apoptosis through interacting with a serine protease Hepsin. Arch Virol. 2005;150:721-741. |
| 9. | Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651-660. |
| 10. | Chan DW, Ng IO. Knock-down of hepatitis B virus X protein reduces the tumorigenicity of hepatocellular carcinoma cells. J Pathol. 2006;208:372-380. |
| 11. | Lai MM, Ware CF. Hepatitis C virus core protein: possible roles in viral pathogenesis. Curr Top Microbiol Immunol. 2000;242:117-134. |
| 12. | Shan Y, Chen XG, Huang B, Hu AB, Xiao D, Guo ZM. Malignant transformation of the cultured human hepatocytes induced by hepatitis C virus core protein. Liver Int. 2005;25:141-147. |
| 13. | Chen RF, Li ZH, Zou SQ, Chen JS. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:71-74. |
| 14. | Koike K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: lessons from animal model studies. Clin Gastroenterol Hepatol. 2005;3:S132-S135. |
| 15. | Han HJ, Jung EY, Lee WJ, Jang KL. Cooperative repression of cyclin-dependent kinase inhibitor p21 gene expression by hepatitis B virus X protein and hepatitis C virus core protein. FEBS Lett. 2002;518:169-172. |
| 16. | Jung EY, Kang HK, Chang J, Yu DY, Jang KL. Cooperative transformation of murine fibroblast NIH3T3 cells by hepatitis C virus core protein and hepatitis B virus X protein. Virus Res. 2003;94:79-84. |
| 17. | Keasler VV, Lerat H, Madden CR, Finegold MJ, McGarvey MJ, Mohammed EM, Forbes SJ, Lemon SM, Hadsell DL, Grona SJ. Increased liver pathology in hepatitis C virus transgenic mice expressing the hepatitis B virus X protein. Virology. 2006;347:466-475. |
| 18. | Zhang X, Dong N, Zhang H, You J, Wang H, Ye L. Effects of hepatitis B virus X protein on human telomerase reverse transcriptase expression and activity in hepatoma cells. J Lab Clin Med. 2005;145:98-104. |
| 19. | Ruggieri A, Murdolo M, Harada T, Miyamura T, Rapicetta M. Cell cycle perturbation in a human hepatoblastoma cell line constitutively expressing Hepatitis C virus core protein. Arch Virol. 2004;149:61-74. |