Published online Sep 21, 2008. doi: 10.3748/wjg.14.5403
Revised: June 23, 2008
Accepted: June 30, 2008
Published online: September 21, 2008
AIM: To investigate the effect and mechanism of action of erlotinib, an epidermal growth factor receptor (EGFR) small molecule tyrosine kinase inhibitor (TKI), in the human pancreatic cancer cell line BxPC-3 both in vitro and in vivo.
METHODS: In vitro, human pancreatic cancer cell line BxPC-3 was exposed to varying concentrations of erlotinib, and its effects on proliferation, cell cycle distribution, apoptosis and the expression of pro- and antiapoptotic factors such as bcl-2, bcl-xl, bax and bak, and the expression of vascular endothelial cell growth factor (VEGF) were measured with 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, flow cytometric analysis, terminal deoxynucleotidyl transferase-mediated nick end labeling assay (TUNEL), and reverse transcription-polymerase chain reaction (RT-PCR). Potential effect of erlotinib on angiogenesis was examined by tube formation assay. Tumor growth suppression was observed in xenografted nude mice with pancreatic cancer in vivo. Immunohistochemical (IHC) staining for EGFR and factor VIII-related antigen was undertaken to detect the microvessel density and VEGF expression in tumor tissue in xenograft nude mice.
RESULTS: Erlotinib, as a single agent, repressed BxPC-3 cell growth in a dose-dependent manner, triggered G1 arrest and induced cell apoptosis, and suppressed capillary formation of endothelium in vitro. Expressions of VEGF were significantly down-regulated at a high concentration of 200 μmol/L, however, the expressions of bcl-2 and bcl-xl were decreased at 50 μmol/L. In vivo, Erlotinib-treated mice demon-strated a reduced tumor volume, weight and microvessel density as compared to the control. IHC staining showed decreased expression of EGFR and RT-PCR had lower VEGF expression in treated mice.
CONCLUSION: The in vitro and in vivo findings provide evidence that BxPC-3 cells are inhibited with erlotinib treatment. Inhibition of EGFR may be a promising adjuvant chemotherapy strategy in pancreatic cancer treatment.
- Citation: Lu YY, Jing DD, Xu M, Wu K, Wang XP. Anti-tumor activity of erlotinib in the BxPC-3 pancreatic cancer cell line. World J Gastroenterol 2008; 14(35): 5403-5411
- URL: https://www.wjgnet.com/1007-9327/full/v14/i35/5403.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5403
| Target genes | Primer sequence | Size (bp) | Annealing temperature (°C) | Cycles | |
| Bcl-2 | Sense | 5'-GGTGCCACCTGTGGTCCACCT-3' | 458 | 54 | 35 |
| Antisense | 5'-CCTCACTTGTGGCCCAGATAGG-3' | ||||
| Bax | Sense | 5'-CTGACATGTTTTCTGACGGC-3' | 289 | 54 | 35 |
| Antisense | 5'-TCAGCCCATCTTCTTCCAGA-3' | ||||
| Bcl-xl | Sense | 5'-TTGGACAATGGACTGGTTG-3' | 765 | 54 | 35 |
| Antisense | 5'-GTAGAGTGGATGGTCAGTG-3' | ||||
| Bak | Sense | 5'-TGAAAAATGGCTTCGGGGCAAGGC-3' | 642 | 54 | 35 |
| Antisense | 5'-TCATGATTTGAAGAATCTTCGTACC-3' | ||||
| GAPDH | Sense | 5'-CATGCCAGTGAGCTTCCCGTT-3' | 408 | 56 | 35 |
| Antisense | 5'-GTGGAGTCTACTGGCGTCTTC-3' | ||||
| VEGF | Sense | 5'-ATGAACTTTCTGCTGTCTTG-3' | 382 | 54 | 35 |
| Antisense | 5'-TGCATGGTGATGTTGGAC-3' |
Pancreatic cancer is one of the most lethal human cancers and continues to be a major unsolved health problem[1,2]. Recently, several orally bioavailable compounds aimed at specific molecular targets have been developed in hopes of improving survival in this dismal disease. Tumor development and progression depend on cellular changes like overexpression of oncogenic tyrosine kinase receptors. Many gastrointestinal tumors, including pancreatic cancer, have been shown to overexpress the epidermal growth factor receptor (EGFR)[3,4]. The overexpression of the EGFR and its ligands correlates with rapidly progressive disease and resistance to chemotherapy.
EGFR is a 170-kDa transmembrane protein with intrinsic tyrosine kinase activity. Stimulation of the EGFR results in activation of multiple intracellular signaling cascades that increase cellular proliferation and prevent programmed cell death[5]. Multiple therapeutic strategies designed to manipulate this receptor have been developed, including specific antibodies and low molecular EGFR tyrosine kinase inhibitors (TKIs). Erlotinib is a small molecule TKI that efficiently blocks EGFR. Preliminary results of a phase III trial of gemcitabine with or without erlotinib in pancreatic cancer revealed a modest improvement in survival with the addition of erlotinib. Treatment with anti-EGFR agents is used as a potential therapeutic strategy for pancreatic cancer, but the mechanisms are not yet precisely understood.
The aim of this study was to investigate the growth inhibitory effects of erlotinib in pancreatic cancer cells in vitro and in vivo, to determine the mechanisms involved and to examine the effects of erlotinib on the regulation of angiogenesis.
Human pancreatic cancer cell lines BxPC-3, obtained from Shanghai Institute of Biochemistry and Cell Biology, and ECV 304, a cell line derived from human umbilical vein endothelial cells from ATCC were maintained in RPMI-1640 (Gibco) medium supplemented with 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. The EGFR-selective TKI erlotinib was provided by DeBioChem (Nanjing, China). The agent was dissolved in DMSO (Sigma) or carboxymethylcellulose sodium at appropriate concentrations for in vitro or in vivo studies, respectively.
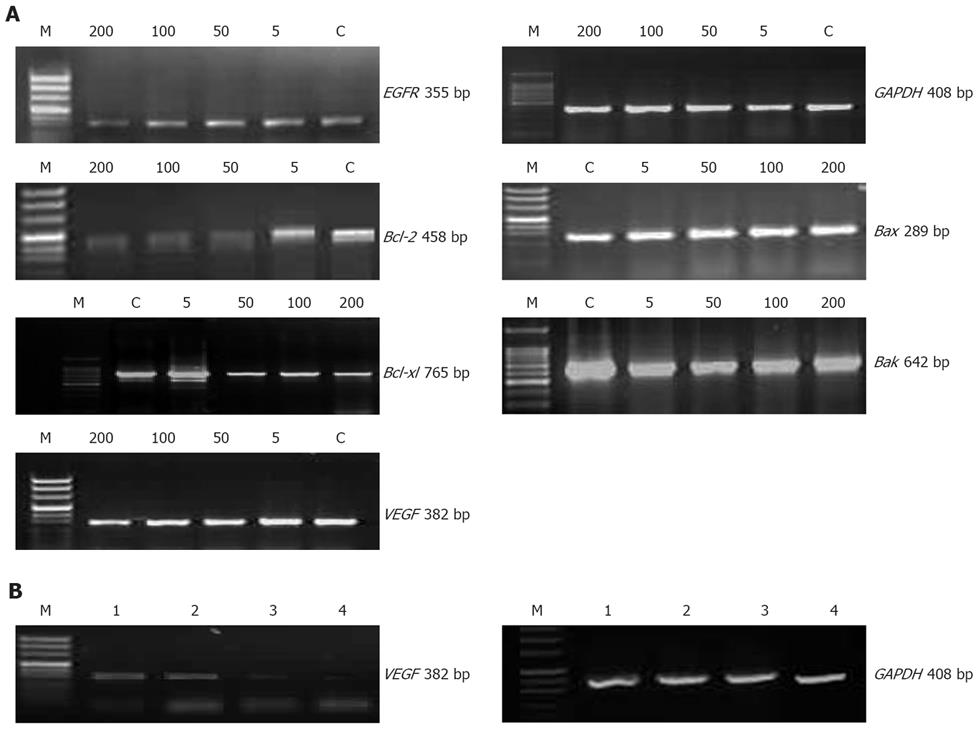
Total cellular RNA was extracted with TRIzol (Life Technologies, INC) following the manufacturer’s instructions. Reverse transcription was performed starting with 2 μg of total RNA, using oligo (dT) primer and other reagents, and procedures contained in the MMulv RT-PCR kit (Promega) to form cDNA. cDNA (2 μL), 2 μL of 50 pmol/L of each primer, 10 mmol/L dNTP Mix 1 μL, 1 μL of Taq DNA polymerase (Sangon, China) were used for PCR analysis. The PCR amplification cycles consisted of denaturation at 94°C for 5 min, 35 cycles of denaturation at 94°C for 60 s, annealing [54°C for bcl-2, bcl-xl, bax, bak, vascular endothelial cell growth factor (VEGF), 56°C for GAPDH] for 60 s, extension at 72°C for 60 s, and a final elongation at 72°C for 10 min. The PCR products were separated on a 1.5% agarose gel, stained with 0.5 mg/mL ethidium bromide, and visualized by UV light. The primer sequences are listed in Table 1.
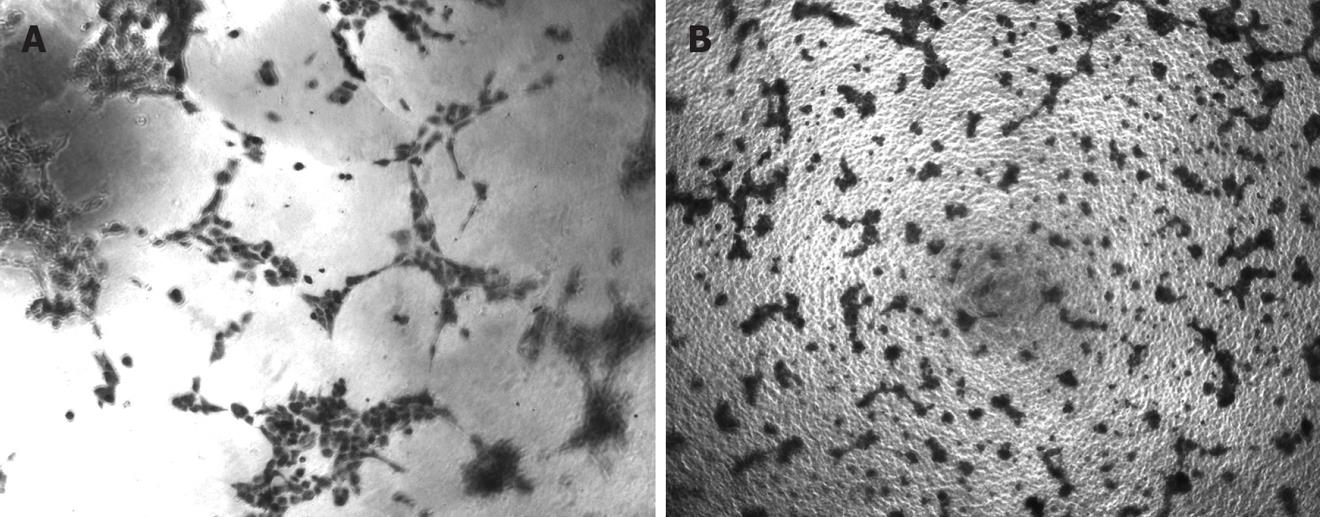
A well established method was used for the process of in vitro angiogenesis assay[6] with a kit from Chemicon (Temecula, California, USA). A 96-well tissue culture plate was coated with Matrigel (50 μL/well). After matrix solution gelled, ECV304 cells were premixed with RPMI-1640 (control), erlotinib (100 μmol/L) and then seed at a concentration of 1 × 104 per well onto the surface of the polymerized gel. Four wells were used for each treatment. After 18 h of incubation at 37°C and 5% CO2, the status of capillary tube formation by ECV304 cells was recorded using a CCD camera attached to an inverted light microscope (40 × objective lens).
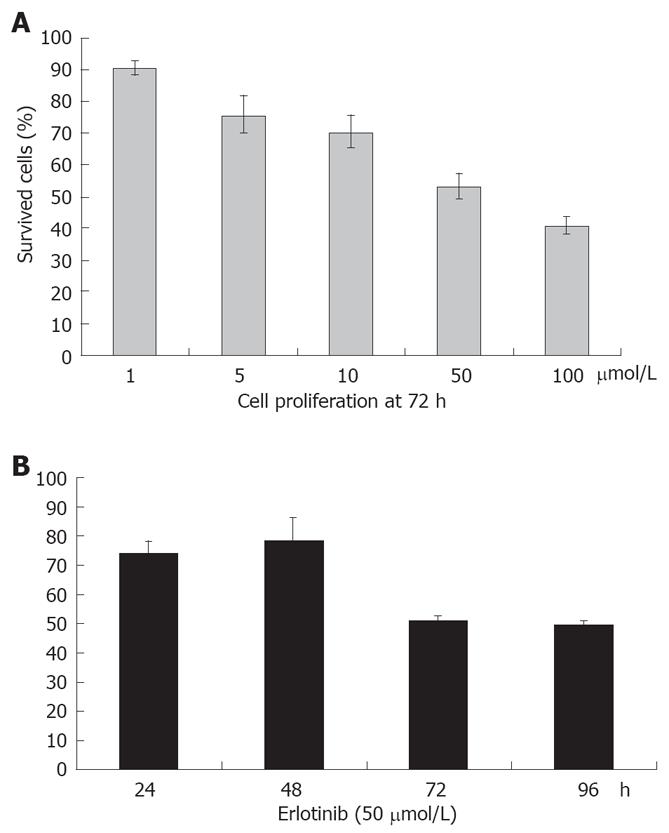
The viability of BxPC-3 cells treated with erlotinib was determined by the standard 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. BxPC-3 cells were plated (5 × 103 per well) in 96-well plates and incubated overnight at 37°C. Erlotinib was dissolved in DMSO and added to the cell culture medium at a concentration not exceeding 0.1% (v/v). The effects of erlotinib on cell proliferation were studied at various concentrations (0, 1, 5, 10, 50 and 100 μmol/L) and at different time points (24, 48, 72 and 96 h) with a certain concentration (50 μmol/L). The MTT assay was done in quadruplicate for each drug concentration used. At appropriate intervals, 100 μg MTT solution was added to each well and incubated for 4 h at 37°C, 5% CO2. The supernatant was removed, and 150 μL of DMSO was then added. Plates were then read at 490 nm wavelength using a microplate reader (BIO-RAD550, USA). Percentage of inhibition was determined by comparing the cell density in the drug-treated cells with that in the untreated cell controls in the same incubation period [percentage of inhibition = (1-cell density of a treated group)/cell density of the control group]. All experiments were repeated three times.
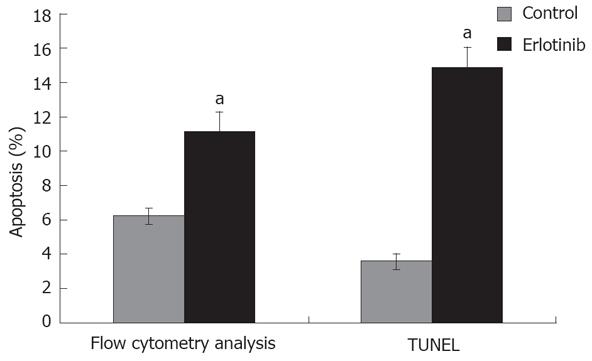
The effects of EGFR TKIs erlotinib on both cell cycle and apoptosis in BxPC-3 cells were analyzed using flow cytometry. Cells were plated into 12-well plates and the following day, erlotinib (50 μmol/L) was added and kept for 48 h. Cell floating in the medium combined with adherent layer were trypsinized and fixed with 2 mL of Citrate buffer for 1 h. Cells were then incubated with RNase A (1500 μL) and stained with propidium iodide (1500 μL). Samples were immediately analyzed by flow cytometry for cell cycle and apoptosis assays. Immunocytochemical (ICC) detection of apoptotic cells was carried out with terminal deoxynucleotidyl transferase-mediated nick end labeling assay (TUNEL), in which residues of digoxigenin-labeled dUTP were catalytically incorporated into the DNA by terminal deoxynucleotidyl transferase II. After treatment with erlotinib (50 μmol/L) for 48 h, slides were fixed and washed thrice in 0.01 mol/L PBS, the following procedures were performed according to the manufacturer instructions (Boster, Wuhan, China). The positive particles of DAB staining were viewed under microscope (Olympus Japan). The number of apoptotic cells was viewed and counted under microscope (40 × objective lens, Olympus Japan) and expressed as the Apoptotic Index (AI = number of apoptotic body/1000 cells).
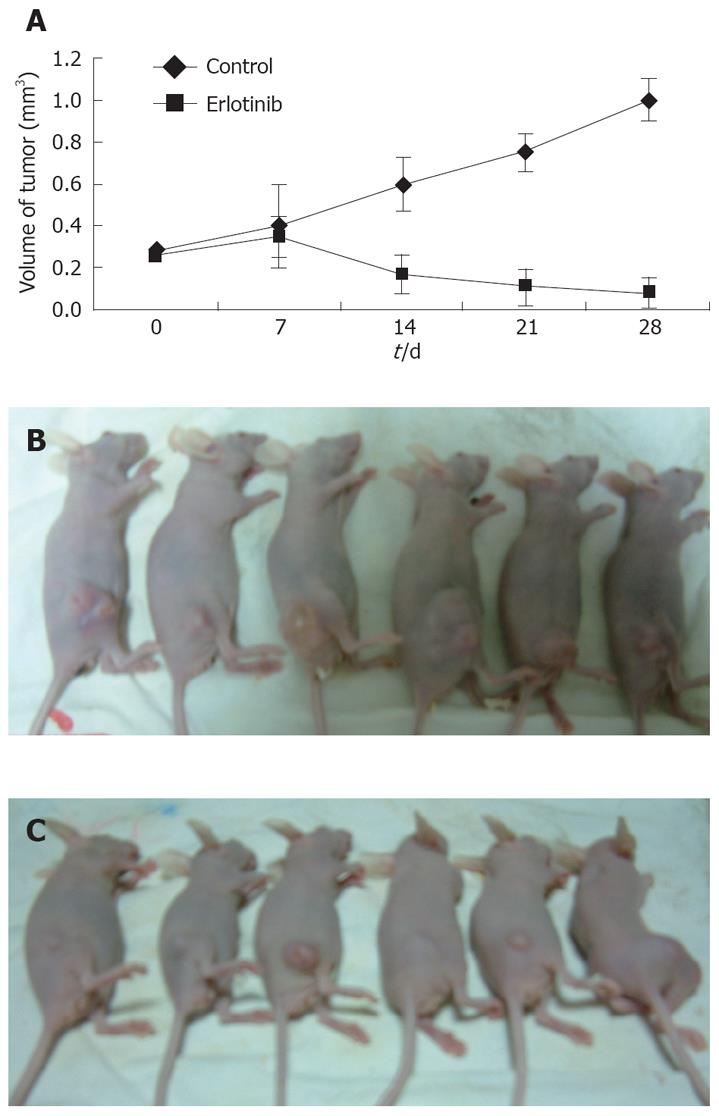
BALB/C nu/nu female mice, aged 4-6 wk, weighing about 20 g, were maintained pathogen free at the Shanghai Experimental Animals Centre of Chinese Academy of Sciences. BxPC-3 cells (1 × 107, suspended in 200 μL of PBS) were implanted s.c. in the hind flank of each mouse. Once palpable tumors were established, animals were randomly divided into two groups so that all groups had similar starting mean tumor volumes of 100-150 mm3. The mice in each group were orally gavaged with the vehicle control (0.5% CMC-Na, n = 6) and erlotinib (100 mg/kg, n = 6) for 4 wk. The tumor size was measured with a linear caliper twice a week up to 4 wk, and the volume was estimated using the equation V = (a × b2)/2, where a is the large dimension and b the perpendicular diameter. After all the mice were sacrificed, part of the tissue was fixed in formalin and embedded in paraffin, and some parts were frozen in liquid nitrogen. Hematoxylin and eosin staining confirmed the presence of tumors. Total mRNA was prepared and RT-PCR analyses were performed as described previously.
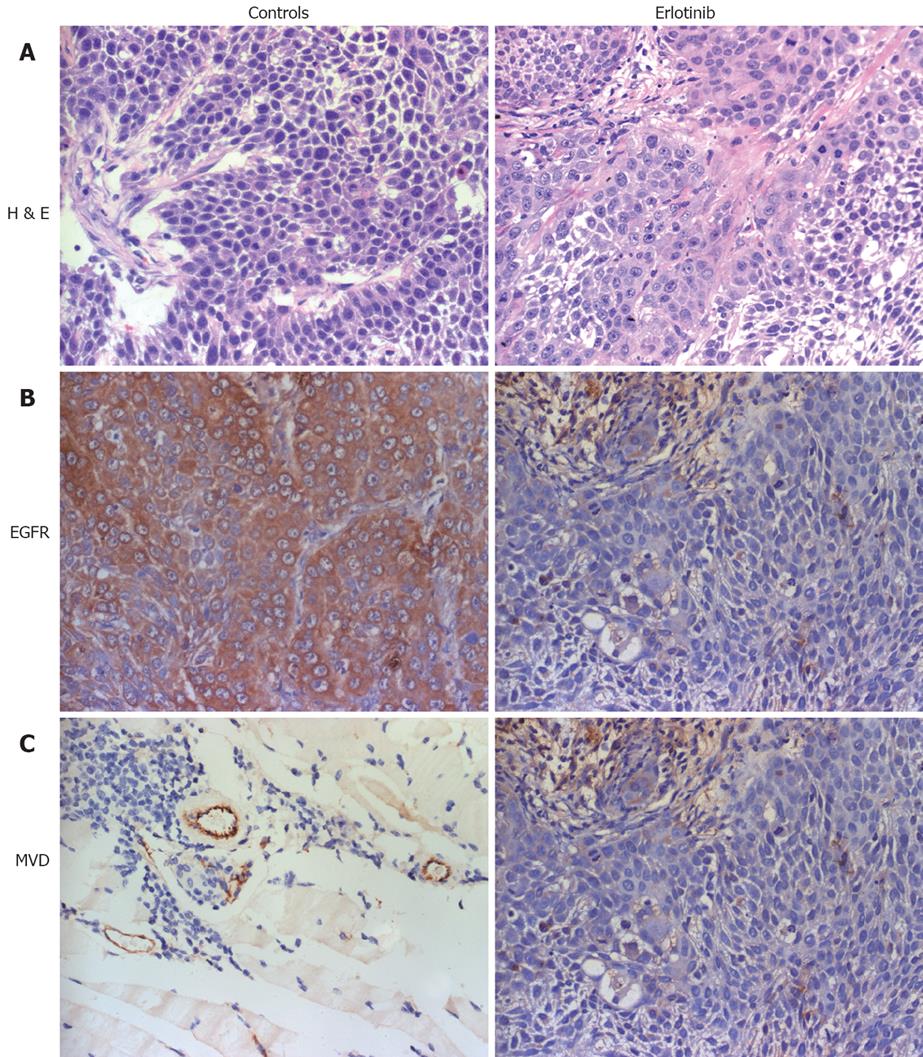
To assess EGFR expression and microvessel density (MVD) in xenograft tumors, rabbit polyclonal anti-EGFR antibody (diluted to 1:100, Boster, Wuhan) and rabbit polyclonal factor VIII antibody (Boster, Wuhan) were used in IHC. Paraffin-embedded tissue sections (4 μm) were dried, deparaffinized, and rehydrated. Endogenous peroxidase was blocked with 3% hydrogenperoxide in ion free water for 30 min. After nonspecific binding sites were blocked with 10% goat serum, slides were incubated at 4°C overnight with 1:100 dilution of primary antibody directed against EGFR and factor VIII followed by a 30-min incubation in a Horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG secondary antibody. Sections were rinsed with PBS and developed with the DAB kit (Boster, Wuhan) and then counterstained with haematoxylin. Each slide was scanned at a low power (× 100) and the area with a higher number of new vessels was identified (hotspot). This region was then scanned at × 400 magnification. For individual tumors, microvessel count was scored by averaging the five field counts[7].
Quantitative results were expressed as mean ± SEM. Statistical analysis was performed using a two-tailed unpaired t test (between two groups) or a one way analysis of variance (ANOVA) (among three or more groups) with the computer software SAS 8.02. P < 0.05 was considered statistically significant.
Overall cell growth of BxPC-3 pancreatic cancer cells treated with erlotinib at different concentrations ranging from 1 to 100 μmol/L was determined by MTT assay. The results showed that erlotinib inhibited cell growth in a dose-dependent manner and significant growth inhibition was demonstrated at 72 h (P < 0.01). A similar result was obtained after erlotinib treatment for 96 h. We also treated BxPC-3 cells with erlotinib at 24 and 48 h. The results showed a trend toward growth inhibition, but it was not statistically significant among different concentrations (P > 0.05). Cell proliferation was again determined by MTT at 24, 48, 72 and 96 h. No significant difference in cell growth was noted between 24 h and 48 h, 72 h and 96 h at a concentration of 50 μmol/L. Percentage of survival cells is illustrated in Figure 1.
To elucidate potential mechanisms of erlotinib-induced growth inhibition previously shown by MTT assay, we examined the distribution of cell cycle and apoptotic cells by flow cytometry and TUNEL. The concentration of the drug was 50 μmol/L which inhibited BxPC-3 cell growth by approximately 53.5% after 3 d of exposure. The results showed that erlotinib led to the accumulation of BxPC-3 cells in G0/G1 phase, thereby decreasing the proportion of cells in the S phase (Table 2). To assess the effects of erlotinib on induction of cell apoptosis of pancreatic cancer cells, we performed a PI apoptosis and TUNEL assay after 48 h. Flow cytometric analysis showed an induction of apoptosis (11%) compared with the control (6%) (P < 0.05), which was further confirmed by TUNEL (AI 14.86 ± 1.20 to 3.60 ± 0.45) (P < 0.05) (Figure 2). The cell cycle alterations and cell apoptosis increase indicated that cell cycle arrest and increase in apoptosis are one of the mechanisms responsible for the antiproliferative action of erlotinib in BxPC-3 cells in vitro.
A nude mouse model of pancreatic cancer was used for the in vivo portion of the study to assess the in vivo antitumor activity of erlotinib. Heterotopic murine pancreatic carcinoma was successfully established in the flank of BALB/C nude mice. Erlotinib at a dose of 100 mg/kg per day was administered to mice bearing established BxPC-3 tumors. The results indicated that erlotinib significantly inhibited tumor growth. As shown in Figure 3, at day 14, the control group of mice showed an increased tumor volume by 114.3% of the initial tumor while the group treated with erlotinib showed a decreased tumor volume by 34.6%. At the end of the study in the BxPC-3 xenograft (day 28), the growth inhibition rate was 74.5% (P < 0.05) in the erlotinib treated group.
EGFR TKIs have been reported to inhibit angiogenesis of pancreatic carcinoma. To determine whether erlotinib suppressed tumor vessel formation in vitro, we examined the effects of erlotinib on the ability of ECV304 to form capillary tube structures. ECMatrix is a solid gel of basement proteins prepared from the Engelbreth Holm-Swarm (EHS) mouse tumor and consists of laminin, collagen type IV, heparin sulphate proteoglycans, entactin, and nidogen. It also contains various growth and proteolytic enzymes that occur normally in EHS tumors. When cultured on ECMatrix, ECV304 cells rapidly aligned and formed tube-like structures (Figure 4A), however, the length of the tubes in the erlotinib treated cells was markedly reduced compared with the control which demonstrated that erlotinib significantly inhibited ECV304 tube formation (Figure 4B).
The EGFR expression pattern in the BxPC-3 tumors was examined by IHC staining. IHC analysis confirmed that the tumor xenograft tissues maintained both cell membrane and cytoplasmic EGFR expression. The expression of EGFR was decreased in the erlotinib treated groups (2.45 ± 0.81) compared with the control (10.65 ± 1.26) (P < 0.05). MVD was also assessed by immunostaining with factor VIII-related antigen in the most intense areas of neovascularization. Representative images of the two groups are shown in Figure 5. MVD of erlotinib treated tumors (1.86 ± 0.43) was significantly lower than that of the control (5.98 ± 1.27) (P < 0.05).
The effect of erlotinib on the expression of EGFR, apoptosis-associated factors such as bcl-2, bak, bax, bcl-xl and VEGF mRNA was determined by RT-PCR. BxPC-3 cells were treated with erlotinib at different concentrations ranging from 5-200 μmol/L for 48 h. The results showed that the expression of EGFR and VEGF mRNA in the BxPC-3 cell line seemed to be down-regulated, the highest suppression was detected at the highest concentration used, 200 μmol/L. Densitometric analysis revealed the relative expression of VEGF at 200 μmol/L concentration of erlotinib (1.2% ± 0.68%) was significantly lower than that of the control (2.67% ± 0.13%) (P < 0.05). Apoptosis-associated gene including bcl-xl and bcl-2 was suppressed by erlotinib in a concentration dependent manner; however, bax appeared to be up-regulated following erlotinib treatment at various concentrations; but it did not apparently affect bak expression. In the tumor xenograft tissues, the expression of VEGF mRNA was significantly lower (almost disappeared) in the erlotinib-treated group compared with that in the control (P < 0.01) (Figure 6).
Treatment options of pancreatic adenocarcinoma are unsatisfactory, and the prognosis of patients with pancreatic cancer is poor. Considering that the cure rate for these patients with surgery alone is low[8], development of potentially effective treatment is urgently needed. Many features of the pancreatic malignant phenotype are associated with the signaling networks that involve the EGFR. EGF and its receptor, EGFR, are over-expression in many human pancreatic cancers[9,10]. Recently, there is increasing evidence demonstrating the therapeutic potential of EGFR blockade in the management of pancreatic cancer and other malignancies[11-13]. EGFR inhibitors have shown activity in clinical trials in pancreatic, colorectal and non-small cell lung cancers[14-16]. Erlotinib is an orally available low-molecular-weight quinazolinamine that acts as a potent and reversible inhibitor of EGFR-TK activity. Single agent activity was observed in patients with non-small cell lung cancer, head and neck carcinoma and ovarian cancer[17-20]. A randomized phase III placebo-controlled trial has shown that the combination of gemcitabine and erlotinib is associated with a modest but statistically significant survival benefit compared with gemcitabine and placebo and this represents an EGFR-targeted agent conferred benefit in addition to chemotherapy[21]. In this study, we evaluated the efficacy of erlotinib, as a single agent, on pancreatic cancer cells grown in vitro and in vivo using a nude mice xenograft model and explored the mechanisms involved as well. In vitro results showed an inhibition efficiency of 53.5% by erlotinib at the concentration of 50 μmol/L in the growth of cultured BxPC-3 pancreatic carcinoma cells as determined by MTT assay. The cell viability of BxPC-3 pancreatic cells was 53.5% with erlotinib treatment at a 50 μmol/L concentration by the MTT assay in vitro. There was no difference between 50 μmol/L and 100 μmol/L concentrations of erlotinib when cells were treated for 48 h (data not shown). We performed cell cycle analyses to characterize the underlying mechanisms of erlotinib’s mode of action. Upon erlotinib treatment for 48 h, the proportion of cells in the G0/G1 phase increased to 73.4%, being significantly higher than that of the control. The antineoplastic effect of erlotinib is not solely due to cell cycle arrest. Induction of apoptosis by EGFR-TK inhibition has recently been reported[22,23]. In our study, we also observed cell apoptosis induced by erlotinib following cell cycle arrest. As we know, Bcl-2 members are crucial regulators of apoptotic cell death. We also checked the expression of anti-apoptotic factors such as bcl-2, bcl-xl as well as pro-apoptotic factors bax and bak. Erlotinib induced a moderate decrease in the expression of bcl-2 and bcl-xl at a concentration of ≥ 50 μmol/L. Our study in vitro provides evidence that the growth of BxPC-3 cells can be suppressed by erlotinib and at least two mechanisms are involved, i.e. cell cycle arrest and apoptosis. We used a nude mouse xenograft model to further evaluate the antitumor efficacy of erlotinib in pancreatic cancer in vivo. Erlotinib was very effective in suppressing the growth of BxPC-3 tumors in a subcutaneous tumor model. Tumor inhibition of approximately 74.5% was observed after 4 wk of treatment with erlotinib at a dose of 100 mg/kg per day. IHC staining of sections of subcutaneously implanted tumors showed constitutively high EGFR expression, however, the mice treated with 100 mg/kg erlotinib daily showed lower levels of EGFR. These results showed that erlotinib as monotherapy has strong antitumor activity in human BxPC-3 xenograft models expressing EGF receptors.
Numerous lines of evidence have shown that angiogenesis plays a significant clinicopathological role in tumors. Although pancreatic cancer is not a grossly vascular tumor, it often exhibits enhanced foli of endothelial cell proliferation. Moreover, several studies have reported a positive correlation between blood vessel density and disease progression in cases of pancreatic cancer, supporting the important role of angiogenesis in this disease[24-26]. EGFR signaling has previously been shown to play a role in angiogenesis[27]. In this study, we used the expression of VEGF, the MVD and tube formation assay to investigate the effects of erlotinib on angiogenesis both in vitro and in vivo. At the molecular level, VEGF is believed to be critical for pancreatic cancer angiogenesis[28]. Erlotinib exhibited a moderate decrease in VEGF expression in vitro, especially at a high concentration (200 μmol/L). Similarly, in xenograft tissues, there were marked reductions in the amounts of VEGF present in the treated group as compared with the control. As a unique tool, an in vitro capillary formation assay has been used to verify specific antiangiogenic activities of many agents with a good correlation to blood vessel formation in vivo[29]. Using this method, we observed that erlotinib as a single agent inhibited capillary tube formation by ECV304. Our immunohistochemical analysis of tumor specimens revealed that the treatment of mice with erlotinib produced a significant decrease in the number of tumor-associated blood vessels (MVD). The decrease in MVD could be attributable to a decrease in endothelial cell proliferation, as we proved in vitro.
In summary, our study showed that blockade of the EGFR signaling pathway by erlotinib has provided significant treatment in the BxPC-3 cell line in vitro and in nude mice and it may have a potential for the treatment of human pancreatic carcinoma. However, it is apparent that the use of a single signal transduction inhibitor cannot antagonize all the potentially relevant survival pathways in pancreatic cancers. As an adjuvant use in chemotherapy, targeting EGFR pathway seems to be a promising approach in the prevention and/or treatment of pancreatic cancer.
The activity of the epidermal growth factor receptor (EGFR), a tyrosine kinase receptor of the ErbB family, is abnormally elevated in most human solid tumors, including pancreatic cancer, and is associated with progression and poor prognosis. There are two main categories of therapeutic strategy for targeting the EGFR pathway, specific anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors (TKIs). Blockage of EGFR activity may lead to growth inhibition in pancreatic cancer.
Molecularly targeted therapies have recently expanded the options available for patients with gastrointestinal tumors. Low weight EGFR TKIs such as erlotinib may play an important role in the treatment of human pancreatic carcinoma. Administration of erlotinib inhibites the BxPC-3 human pancreatic cancer cell line growth and induces antiangiogenic effect both in vitro and in vivo.
Small-molecule inhibitors of the EGFR tyrosine kinase, such as erlotinib, have shown promise in phase III trials in non-small-cell lung cancer (NSCLC). But the anticancer mechanism has not been clearly elucidated especially in gastrointestinal tumors, including pancreatic cancer. The results indicate that the blockade of EGFR may provide a rationale for translating this therapeutic strategy into a clinical setting in some pancreatic cancer patients.
This study showed that blockade of the EGFR signaling pathway by erlotinib suppressed the BxPC-3 human pancreatic cancer cell line growth and induced antiangiogenic effects both in vitro and in vivo. As an adjuvant used in chemotherapy, targeting the EGFR pathway seems to be a promising approach in the prevention and or treatment of pancreatic cancer.
The epidermal growth factor (EGF) receptor (or ErbB1) and the related ErbB4 are transmembrane receptor protein tyrosine kinases which bind extracellular ligands of the EGF family. ErbB2 and ErbB3 are “co-receptors” structurally related to ErbB1/ ErbB4, but ErbB2 is an “orphan” receptor and ErbB3 lacks tyrosine kinase activity. They transduced biological signals from the extracellular to the intracellular compartment. These families of ligands and receptors have been firmly linked to proliferative signaling and oncogenesis.
In this paper, the effect of erlotinib on the proliferation and apoptosis of the human pancreatic cancer cell line BxPC-3 was studied. A dose-dependent inhibition of BxPC-3 cell growth in vitro, with a block of G1-S transition, and rise in apoptosis were observed in erlotinib-treated cells. A decrease in tumor volume and microvessel density was also found in erlotinib-treated nude mice carrying BxPC-3 cell xerography. This is a very interesting manuscript with some minor points needing further addressing.
Peer reviewers: Francesco Feo, Professor, Dipartimento di Scienze Biomediche, Sezione di Patologia Sperimentale e Oncologia, Università di Sassari, Via P, Manzella 4, Sassari 07100, Italy; Sharon DeMorrow, Division of Research and Education, Scott and White Hospital and The Texas A&M University System, Health Science Center College of Medicine, Temple, Texas 76504, United States
S- Editor Li DL L- Editor Ma JY E- Editor Lin YP
| 2. | MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004;5:541-549. |
| 3. | Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M. Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002;62:5611-5617. |
| 4. | Solorzano CC, Baker CH, Tsan R, Traxler P, Cohen P, Buchdunger E, Killion JJ, Fidler IJ. Optimization for the blockade of epidermal growth factor receptor signaling for therapy of human pancreatic carcinoma. Clin Cancer Res. 2001;7:2563-2572. |
| 5. | Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926-2935. |
| 6. | Taraboletti G, Giavazzi R. Modelling approaches for angiogenesis. Eur J Cancer. 2004;40:881-889. |
| 7. | Ciardiello F, Caputo R, Damiano V, Caputo R, Troiani T, Vitagliano D, Carlomagno F, Veneziani BM, Fontanini G, Bianco AR. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546-1556. |
| 8. | Smeenk HG, Tran TC, Erdmann J, van Eijck CH, Jeekel J. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94-103. |
| 9. | Poch B, Gansauge F, Schwarz A, Seufferlein T, Schnelldorfer T, Ramadani M, Beger HG, Gansauge S. Epidermal growth factor induces cyclin D1 in human pancreatic carcinoma: evidence for a cyclin D1-dependent cell cycle progression. Pancreas. 2001;23:280-287. |
| 10. | Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305-309. |
| 11. | Giaccone G, Gallegos Ruiz M, Le Chevalier T, Thatcher N, Smit E, Rodriguez JA, Janne P, Oulid-Aissa D, Soria JC. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res. 2006;12:6049-6055. |
| 12. | Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927. |
| 13. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-3074. |
| 14. | Welch SA, Moore MJ. Erlotinib: success of a molecularly targeted agent for the treatment of advanced pancreatic cancer. Future Oncol. 2007;3:247-254. |
| 15. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. |
| 16. | Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, Skarin AT, Meyerson M, Holmes AJ, Borras AM. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760-766. |
| 17. | Plestina S, Samarzija M, Plestina S, Chalfe N, Zuljevic E. [Erlotinib in non-small cell lung cancer: experience of clinical hospital for lung diseases Jordanovac]. Lijec Vjesn. 2007;129:387-390. |
| 18. | Thomas F, Rochaix P, Benlyazid A, Sarini J, Rives M, Lefebvre JL, Allal BC, Courbon F, Chatelut E, Delord JP. Pilot study of neoadjuvant treatment with erlotinib in nonmetastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:7086-7092. |
| 19. | Gordon AN, Finkler N, Edwards RP, Garcia AA, Crozier M, Irwin DH, Barrett E. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785-792. |
| 20. | Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77-85. |
| 21. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. |
| 22. | Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777-783. |
| 23. | Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355-5362. |
| 24. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. |
| 25. | Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439-1447. |
| 26. | Khorana AA, Hu YC, Ryan CK, Komorowski RA, Hostetter G, Ahrendt SA. Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg. 2005;9:903-911. |
| 27. | Azuma M, Tamatani T, Fukui K, Yuki T, Yoshida H, Bando T, Hoque MO, Kamogashira T, Ogino K, Nishino N. Identification of EGF as an angiogenic factor present in conditioned medium from human salivary gland adenocarcinoma cell clones with varying degrees of metastatic potential. Cancer Lett. 1994;84:189-198. |
| 28. | Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297-324. |
| 29. | Weiss RH, Marshall D, Howard L, Corbacho AM, Cheung AT, Sawai ET. Suppression of breast cancer growth and angiogenesis by an antisense oligodeoxynucleotide to p21(Waf1/Cip1). Cancer Lett. 2003;189:39-48. |