Published online Sep 14, 2008. doi: 10.3748/wjg.14.5349
Revised: July 28, 2008
Accepted: August 4, 2008
Published online: September 14, 2008
We present a case report of a patient with a suspicious ileal carcinoid tumour. Clinical examination as well as computer tomography (CT) scan suggested a tumour. Octeotride scan showed uptake in the same bowel loop reported as pathological in CT. The patient underwent surgery and biopsy which reported Crohn’s disease (CD). The interest in the case is due to the fact that this is, to the best of our knowledge, the second report of Crohn’s disease as a cause of false positive octeotride scan. Unfortunately, no somatostatin receptors could be found in the sample, so further studies should be performed.
- Citation: Fernandez A, Tabuenca O, Peteiro A. A “false positive” octreoscan in ileal Crohn’s disease. World J Gastroenterol 2008; 14(34): 5349-5352
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5349.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5349
Somatostatin receptors (SS-Rs) are membrane glycoproteins spreading over a large number of body tissues and can be found in normal and pathological conditions[1]. Many benign and malignant tumours over express SS-R in their membranes especially in neuroendocrine tumours (NETs) but there are other benign conditions showing an increase number of SS-Rs, such as granulomatous or inflammatory disease[2].
Octeotride is an analogue whose molecule is a shortened version of somatostatin’s, from 8 to 14 aminoacides, sharing its active nuclei and allowing the 111Indium-DPTA molecule to bind to the N-terminal (d-phe).
Thus, 111In- DTPA-D- Phe1- octreótido (111In- DTPAOC) is the labelled form of octreotide. There are 5 subtypes of SS-R, and somatostatin shows a high affinity for all of them. However, octeotride has a high affinity for only SS-R 2 subtype and a low affinity for SS-R 3 and SS-R 5 subtypes. Therefore, pathological conditions over expressing these receptors are able to be imaged in 111In- DTPAOC scans.
The sensitivity of 111In- DTPAOC in detecting these pathological conditions is variable being very high in carcinoid tumour (86%)[3]. Uptake in other tissues can lead to false-positive findings when studying suspected NETs. Only one case of Crohn’s disease (CD) has been recently reported as a cause of false positive scan[4].
We report a case of a false positive 111In- DTPAOC scintigraphy in a patient with CD mimicking an ileal carcinoid tumour. A discussion about SS-R potential alterations in CD was included.
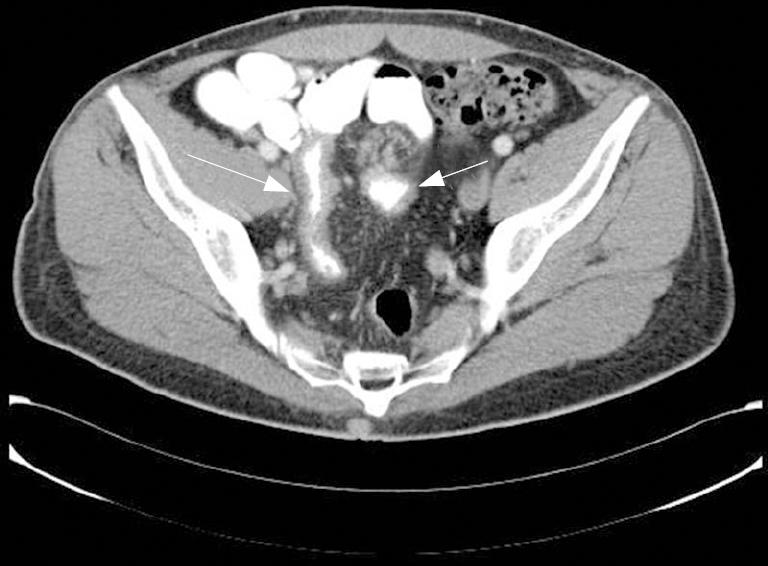
A 40-year-old man was admitted to our outpatient clinic with an 8-mo history of dull abdominal pain and weight loss of 20 kg, but no other gastrointestinal symptoms. In terms of past medical or surgery history, the patient denied of smoking or other diseases. Physical examination only revealed a painful site in the right lower quadrant of the abdomen and a body temperature of 37.3°C. Laboratory test only revealed an increased erythrocyte sedimentation rate of 35 mm/h (normal range: 0-25 mm/h) and C-reactive protein rate of 2.37 mg/dL (normal range: 0-0.5 mg/dL). A computer tomography (CT) scan was performed (Figure 1) and showed thickening of the ileum distal wall and the presence of a 4 cm solid mass with irregular border adjacent to the affected ileum, conditioning retraction, with prominent mesenteric lymph nodes. All these findings suggested a carcinoid tumour.
Further explorations were performed. Colonoscopy with ileoscopy (at least 15 cm of the distal ileum was explored) showed an irregular ileal mucosa with erythema, irregular nodular areas and marked stiffness, suggesting an infiltrative process. Ten ileal biopsies were taken and showed unspecific inflammatory changes without evidence of malignancy, granulomas or histological features suggestive of carcinoid tumour or CD. A 24-h urine collection for 5-hydroxyindolacetic acid (5-HIAA) showed a normal result of 3.3 mg/24 h (normal range < 10 mg/24 h).
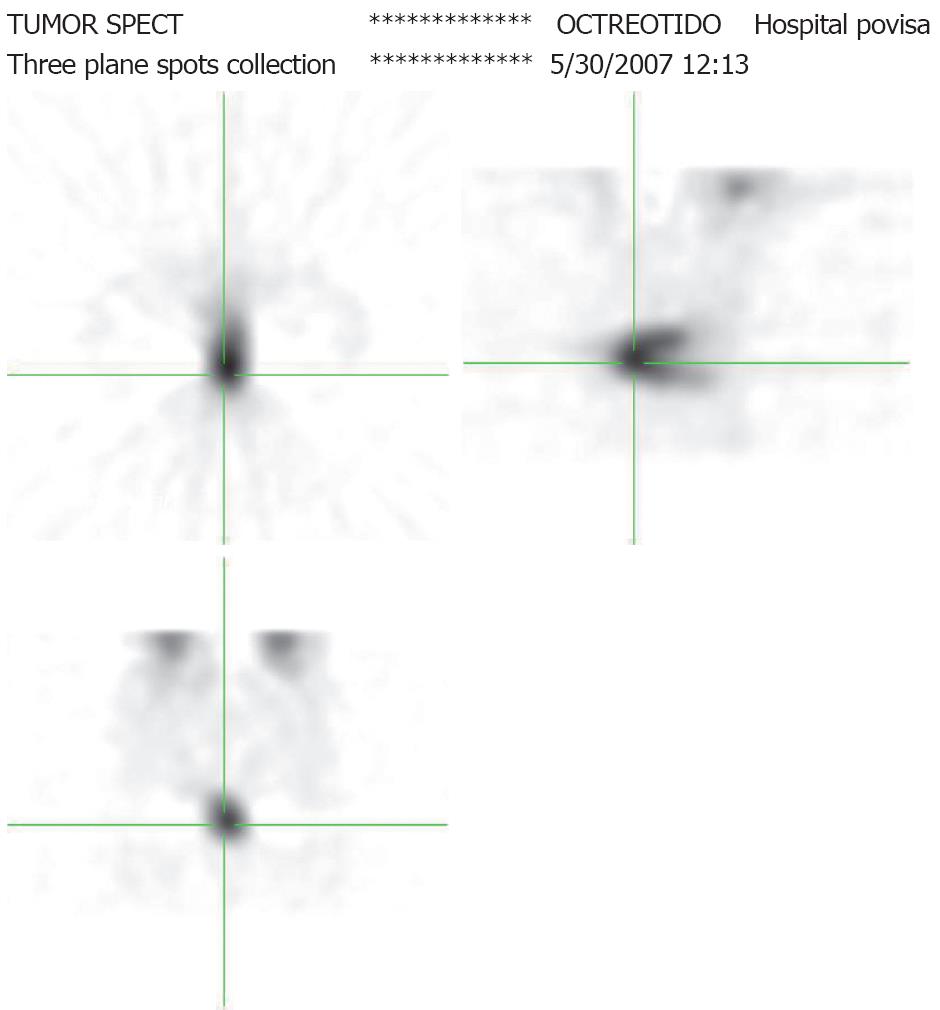
Due to the low sensitivity of the 24-h 5-HIAA test and the high suspicion of carcinoid tumour, an 111In- DTPAOC scan was performed. The patient was injected with 3mCi 111In- DTPAOC. Total body images were obtained at 4 and 24 h, and an abdominal SPECT was also performed at 14 h. The images showed pathological uptake in central and right pelvic fossae at 4 and 24 h (Figure 2). SPECT showed a C-shape uptake in the sagittal plane that matches with the thickened loop on CT. No uptake was observed in the solid mass. The scan was reported as a highly suspicious carcinoid tumour.
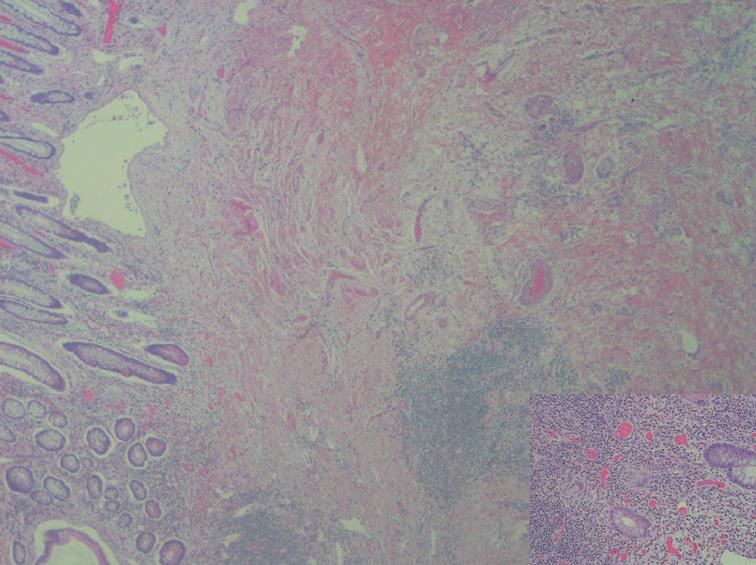
With all these results, laparoscopy with curative intention was performed. An inflammatory mass involving the distal ileon was found at surgery, and a distal small bowel resection and right hemicolectomy with lateral anastomosis were performed. Histological analysis of macroscopically-affected segments and specimens of non-macroscopically-involved segments was carried out. Thickened serosa and shortened mesentery could be grossly observed in the involved terminal ileum (20 cm), resulting in a corrugated bowel contour in the terminal portion of the small bowel. These changes could probably mimic the impression on CT-scan to an abdominal mass. The mesenteric lymph nodes were enlarged measuring 8 mm in the larger diameter. The detailed macroscopic examination failed to show any tumour mass. Photo of gross specimen neither was nor included. Other gross findings of significance included stricture formation, fissuring, cobblestone appearance (discontinuous involvement with transmural spread) with an intervening normal or edematous mucosa. The main microscopic features were ulceration, fissure, non caseating sarcoid-like granuloma and lymphoid aggregates. The regional lymph nodes showed sinusal dilatation and lymphoid hyperplasia. Chromogranin stain was not performed.
Intestinal tissue sections were routinely fixed in 10% neutral formalin and embedded in paraffin. Immunohistochemical staining for Dako® polyclonal antibodies against human somatostatin protein was performed with the standard avidin-biotin method. The slide-mounted tissue sections were allowed to reach room temperature and incubated for 60 min at a solution of 1:900, using saponic to antigenic recuperation. Somatostatin immunohistochemical expression was undetectable in the veins or arteries of inflamed and non inflamed control intestinal tissue sections. No concordance was reached in immunohistochemistry/scintigraphy somatostatin receptor. No areas of dysplasia or carcinoma were identified.
In the absence of features other than the typical appearance of CD, the pathologic diagnosis of Crohn’s ileitis was made (Figure 3).
After surgery, azathioprine was started for prevention of postoperative recurrence. The patient underwent a complete clinical recovery and 6 mo later he was asymptomatic. A surveillance/follow-up colonoscopy did not show any abnormality and inflammatory signs of recurrence.
There are many cases of carcinoid tumour misdiagnosed as CD only discovered when treatment is not effective or surgery is performed. It was reported that approximately 2.3% of patients with ileal carcinoid are first diagnosed and treated as CD[5]. However, the reverse is a very uncommon situation.
In our case, the clinic features as well as the image of CT-scan strongly suggested a carcinoid tumour. In order to confirm the suspected disease, 111In- DTPAOC scan was performed and showed a C-shape uptake in the bowel loop that corresponded to that reported as pathologic in CT, but there was no uptake in the solid mass. The patient underwent surgery and the final pathologic report was CD. The mass that did not show uptake was reported as inflammatory.
111In- DTPAOC is a radio-labelled octreotide analogue that binds to SS-R expressed in cell membranes. Many benign and malignant diseases overexpress SS-R and thus, can be imaged with this radioligand[6]. Carcinoid tumour, one of the malignant tumours, shows more uptakes in the bowel loop due to its high density of SS-R. Reported values for the detection of known carcinoid tumour localizations vary from 80% to nearly 100%[1]. Uptake is related to SS-R density and even tumours smaller than 1 cm in diameter can be detected. This is the reason why somatostatin receptor scintigraphy (SRS) plays a central role in locating and assessing the primary gastroenteropancreatic NET[7] and has a marked effect in the clinical management of these malignancies[8,9]. However, 12% of SRS examinations result in false-positive localization of NET, understanding a false-positive as an uptake not related with the tested pathology. Renal parapelvic cysts, accessory spleens, ventral hernias, thyroid or breast disease are the most frequent cases of false positive localizations[10]. SS-R is over expressed in activated peripheric lymphocytes and macrophages in granulomatous and inflammatory diseases, allowing obtaining images[11,12].
In normal bowel, SS-R is expressed in gastrointestinal mucosa, peripheral nervous system and lymphoid tissue. Several tumours, such as carcinoid tumour, express SS-R in peritumoral veins[13]. In intestinal inflammatory disease, a high density of SS-R is detected by autoradiography in intestinal intramural veins but not in normal tissues. SS-R is seen in small muscular veins of submucosa, tunica muscularis and subserosa, and the thickness of veins is labelled. These veins are histologically normal and seldom have a minimal lymphocyte infiltration although label intensity does not seem to depend on them as the surrounding tissues are infiltrated with these cells and do not show labelling[14-16].
Usually no 111In- DTPAOC uptake is found in inflammatory bowel disease. There is only one recent report of 111In- DTPAOC uptake in Crohn’s disease in literature although there is no evidence that SS-R is determined[4]. In our case, the samples were studied to assess the existence of SS-R. Immunohistochemical staining of somatostatin receptors was performed and somatostatin immunohistochemical expression was undetectable in the veins or arteries of inflamed and non inflamed control intestines.
Unfortunately, SS-R could not be found in the tissues studied, so we could not explain this abnormal uptake. No concordance was reached in immunohistochemistry/scintigraphy somatostatin receptor, possibly as a consequence of somatostatin receptor heterogeneity. Further studies should be made to assess the possible causes of uptake in CD.
However, there are several reports of small carcinoid tumours (a few millimetres in diameter ) found in patients with CD, suggesting a correlation between the pathogenesis of both disorders[17]. It has been theorized that inflammation creates a favourable environment for the development of carcinoid tumour, although most of the carcinoid tumours reported are found in non-inflamed bowel. Other theories include the hyperstimulation of enteroendocrine cells by inflammation[18] and the role of proinflammatory cytokines such as PTHrP or IL-6[19].
We would like to encourage physicians to be aware of CD as a cause of false positive results when performing 111In- DTPAOC scans.
Peer reviewer: Gert De Hertogh, Ph.D, Department of Morphology and Molecular Pathology, University Hospitals KULeuven, Minderbroedersstraat 12, Leuven 3000, Belgium
S- Editor Li DL L- Editor Wang XL E- Editor Zhang WB
| 1. | Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med. 2000;41:1704-1713. |
| 2. | van Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism. 1996;45:86-87. |
| 3. | Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84-91. |
| 4. | Marko J, Lamba R, Miller F, Buchman A, Spies S, Nikolaidis P. OctreoScan positive Crohn's disease mimicking an ileal carcinoid tumor. J Clin Gastroenterol. 2008;42:66-68. |
| 5. | Hsu EY, Feldman JM, Lichtenstein GR. Ileal carcinoid tumors stimulating Crohn's disease: incidence among 176 consecutive cases of ileal carcinoid. Am J Gastroenterol. 1997;92:2062-2065. |
| 6. | Warner RR, O'dorisio TM. Radiolabeled peptides in diagnosis and tumor imaging: clinical overview. Semin Nucl Med. 2002;32:79-83. |
| 7. | Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, Hawkins R, McNicol AM, Reed N, Sutton R. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54 Suppl 4:iv1-iv16. |
| 8. | Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, Mignon M, le Guludec D. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853-858. |
| 9. | Termanini B, Gibril F, Reynolds JC, Doppman JL, Chen CC, Stewart CA, Sutliff VE, Jensen RT. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112:335-347. |
| 10. | Gibril F, Reynolds JC, Chen CC, Yu F, Goebel SU, Serrano J, Doppman JL, Jensen RT. Specificity of somatostatin receptor scintigraphy: a prospective study and effects of false-positive localizations on management in patients with gastrinomas. J Nucl Med. 1999;40:539-553. |
| 11. | Vanhagen PM, Krenning EP, Reubi JC, Kwekkeboom DJ, Bakker WH, Mulder AH, Laissue I, Hoogstede HC, Lamberts SW. Somatostatin analogue scintigraphy in granulomatous diseases. Eur J Nucl Med. 1994;21:497-502. |
| 12. | Tabuenca Dopico O, Gutierrez Mendiguchia C, Rego Iraeta A. [Incidental diagnosis of lymph node tuberculosis in an 111-indium-octreotide scintigraphy during a study of acromegaly]. Rev Esp Med Nucl. 2006;25:193-197. |
| 13. | Reubi JC, Horisberger U, Laissue J. High density of somatostatin receptors in veins surrounding human cancer tissue: role in tumor-host interaction? Int J Cancer. 1994;56:681-688. |
| 14. | Reubi JC, Mazzucchelli L, Laissue JA. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology. 1994;106:951-959. |
| 15. | Denzler B, Reubi JC. Expression of somatostatin receptors in peritumoral veins of human tumors. Cancer. 1999;85:188-198. |
| 16. | Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836-846. |
| 17. | West NE, Wise PE, Herline AJ, Muldoon RL, Chopp WV, Schwartz DA. Carcinoid tumors are 15 times more common in patients with Crohn's disease. Inflamm Bowel Dis. 2007;13:1129-1134. |
| 18. | Le Marc'hadour F, Bost F, Peoc'h M, Roux JJ, Pasquier D, Pasquier B. Carcinoid tumour complicating inflammatory bowel disease. A study of two cases with review of the literature. Pathol Res Pract. 1994;190:1185-1192; discussion 1193-1200. |
| 19. | Barhoum M, Hutchins L, Fonseca VA. Intractable hypercalcemia due to a metastatic carcinoid secreting parathyroid hormone-related peptide and interleukin-6: response to octreotide. Am J Med Sci. 1999;318:203-205. |